1. Background
Respiratory distress syndrome (RDS) is considered one of the most common causes of morbidity in premature neonates. Diagnosis in most cases is based on both clinical and radiographic findings combined, with clinical manifestations, such as grunting, intercostal and subcostal retraction, nasal flaring, and cyanosis, which occur shortly after birth. Surfactant administration is referred to as the cobblestone in the treatment of RDS, which is associated with an increase in pulmonary volume, functional residual capacity (FRC) stabilization, improved ventilation to perfusion ratio, improved oxygenation, and reduced prevalence of air leakage syndromes. The administration of nasal continuous positive airway pressure (nCPAP) and surfactant prescription are currently recognized as the building blocks in the RDS, which require the application of intubation and installation of a tracheal tube to administer surfactant (1-4).
There is no doubt that endotracheal intubation using a laryngoscope is one of the most common methods used in the neonatal intensive care unit (5-7). Due to the pain and anxiety that endotracheal intubation causes for the neonate and since it might cause complications, such as penetration in the trachea and hypopharynx, pseudo-diverticulum, hemorrhage, mucosal necrosis, vocal cord injury, laryngeal edema, and dislocation of the arytenoid cartilages, which will be more acute if the newborn is awake, alternative methods have been suggested (8-11). Using a laryngoscope is associated with pain (perceived by the neonate after 24 weeks of gestation) and anxiety in a newborn. Moreover, neonates less than 6 months of age have limited ability to control pain by reducing nerve pathways, which results in an intensified experience of pain (3, 9, 12).
The hemodynamic effects of pain cause an average rise in blood pressure of up to 33 mmHg and a heart rate increase of more than 30 beats per minute compared to the baseline number. These effects result from the release of catecholamines and cortisol, which are in turn associated with changes in cerebral blood flow velocity. These physio-humoral changes can also be accompanied by a sudden drop in heart rate and blood pressure by stimulating the vagus nerve while administering the endotracheal tube. In addition, these sudden changes in a neonate’s heart rate, blood pressure, and increased need for oxygen (due to a sudden drop in FRC resulting from vocal cord dysfunction) can lead to complications, such as hypoxia-asphyxia, intraventricular hemorrhage (IVH), and intracranial hemorrhage (5, 6, 9, 13). During the implantation of a tracheal tube, an increase in pressure was observed in the anterior fontanelle, which might represent a rise in intracranial pressure (14).
For the purpose of avoiding intubation while administering the surfactant through INSURE method, alternative methods, such as using nebulizers, laryngeal mask airway, administering surfactant into nasopharynx during childbirth, and intraamniotic surfactant administration for women with the risk of preterm birth, have been suggested (15).
The introduction of vibrating nebulizers based on the vibration of mesh plates with kinetic inertia created by piezoelectricity has developed a promising prospect of producing droplets with a diameter of 3 μm capable of penetrating the deepest structure of the respiratory tree. This system is based on a plate with a diameter of 5 mm having 1000 5-μm holes moving by the kinetic energy generated by piezoelectric rings vibrating at a rate of 128,000 vibrations per second (16).
2. Objectives
Following the conducted studies, especially associated with aerosolized surfactant administration and the capabilities of mesh nebulizer machines, it was decided to administer Survanta through aerosolization using mesh nebulizer approach as a research study in comparison to the standard method of INSURE in neonates with RDS.
3. Methods
3.1. Design and Setting
This randomized controlled trial (RCT) study was conducted on neonates with a gestational age of 28 - 32 weeks who needed surfactant administration due to RDS in Beheshti Medical Center in Isfahan, Iran, within June 2019 to June 2021. The neonates participating in the study showed clinical signs of RDS, such as tachypnea, intercostal retraction, nasal flaring, and grunting, and chest radiographs demonstrating RDS requiring surfactant administration during the first 2 hours of life. Surfactant was administered if, despite receiving nCPAP with continuous distending pressure (CDP) ≥ 5 cm H2O, newborns needed the fraction of inspired oxygen (FiO2) ≥ 0.4 to maintain oxygen saturation (SpO2) in the right hand within the range of 89 - 95% (17). The exclusion criteria included perinatal asphyxia (characterized by an Apgar score ≤ 3 in the 5th minute of life, umbilical cord pH < 7, and umbilical cord bicarbonate < 12 mEq/L), congenital anomalies, and receiving surfactant over 2 hours after birth (18, 19).
3.2. Patients
The participants of the current study included 50 neonates divided into two groups (i.e., control and intervention), each consisting of 25 subjects with the gestational age of 28 - 32 weeks needing surfactant administration due to RDS. For randomization, the patients with even file numbers were assigned to the control group, and those with an odd file number were assigned to the intervention group until the recruitment phase was completed.
3.3. Interventions
All neonates in both groups underwent bubble CPAP and, if needed to maintain FiO2 ≥ 0.4, they were administered 100 mg/kg of Survanta under CDP ≥ 5 cm H2O to maintain SpO2 within the range of 89 - 95% in the right hand for more than 30 minutes (20).
In the control group (i.e., the INSURE group), the neonates were intubated using a double-lumen endotracheal tube (Portex, Smiths Medical, UK) after separation from nCPAP and administration of 0.1 mg/kg of intravenous morphine. Then, Survanta was administered in four separate doses after the stabilization of vital signs (appropriate and symmetrical hearing of pulmonary sounds and SpO2 within the range of 89 - 95%) and each administration of positive pressure ventilation for at least 1 minute in a way that, in total, the intervention was completed in less than 10 minutes (20).
In the intervention group (i.e., the aerosolized group), while neonates were under nCPAP, the Aerogen Solo was attached to the inspiration arm using a T-piece, and Survanta was inserted into the chamber according to the neonate’s weight, followed by the activation of Aerogen Solo for 30 minutes (21, 22).
In both groups, if a newborn’s need for FiO2 greater than 0.4 was stable to maintain SpO2 within an acceptable range, the neonate was readministered Survanta by INSURE method 6 hours after the previous administration. In total, if needed, a treatment course of up to four Survanta administrations would be prescribed (20). In addition, an arterial blood gas test was taken before and 3 hours after surfactant administration to determine the arterial/alveolar oxygen (a/APO2) ratio (23, 24).
The invasive ventilation began after the occurrence of any of the following indicators: (1) Requiring FiO2 > 0.7 to maintain SpO2 within the range of 89 - 95% (25); (2) experiencing apnea more than three times requiring stimulation or ventilation with bags and masks (26); (3) respiratory failure defined by pH < 7.2 and the partial pressure of carbon dioxide > 65 mmHg (27).
During respiratory management, if a neonate needs FiO2 at levels below 50% to maintain SpO2 within acceptable limits for more than 4 hours, CDP would gradually decrease by 1 to 2 cm H2O at a time, and nCPAP was removed at CDP = 4 cm H2O and FiO2 < 30% (26).
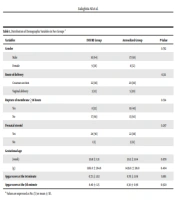
Brain ultrasound imaging was performed on the neonates on the third, seventh, and fourteenth days after birth to evaluate IVH and periventricular leukomalacia (PVL). The demographic characteristics (Table 1) questionnaire recorded the duration of noninvasive respiratory support, prescribed surfactant doses, need for mechanical ventilation, duration of supplemental O2 need, and pneumothorax.
| Variables | INSURE Group | Aerosolized Group | P-Value |
|---|---|---|---|
| Gender | 0.762 | ||
| Male | 16 (64) | 17 (68) | |
| Female | 9 (36) | 8 (32) | |
| Route of delivery | 0.311 | ||
| Cesarean section | 22 (88) | 20 (80) | |
| Vaginal delivery | 3 (12) | 5 (20) | |
| Rupture of membrane ≥18 hours | 0.724 | ||
| Yes | 8 (32) | 10 (40) | |
| No | 17 (68) | 15 (60) | |
| Prenatal steroid | 0.297 | ||
| Yes | 24 (96) | 22 (88) | |
| No | 1 (1) | 3 (12) | |
| Gestational age (wk) | 29.8 ± 1.31 | 30.3 ± 1.04 | 0.078 |
| xBirth weight (g) | 1362.6 ± 364.6 | 1439.6 ± 316.0 | 0.404 |
| Apgar score at the 1st minute | 6.72 ± 1.02 | 6.76 ± 1.09 | 0.861 |
| Apgar score at the 5th minute | 8.40 ± 1.15 | 8.38 ± 0.96 | 0.920 |
a Values are expressed as No. (%) or mean ± SD.
This research study was registered on the Iranian Registry of Clinical Trials (reference no.: IRCT20120728010430N10).
3.4. Main Outcome Measures
The primary objective of the present study was to assess the effect of aerosolized surfactant administration on RDS treatment in neonates with 28 - 32 weeks of gestation.
4. Results
As shown in Table 2, the a/APO2 ratio showed improvement after surfactant administration through nebulization. However, neither the a/APO2 ratio before and after Survanta administration in both control and intervention groups nor the need to administer a second dose of surfactant showed any significant difference. Similarly, no significant difference was observed between the duration of required nCPAP administration and the prevalence of chronic lung disease (CLD), between the need for mechanical ventilation and the prevalence of pneumothorax, or between the prevalence of grades III and IV of IVH and death up to 28 days of birth.
| Aerosolized Group | INSURE Group | P-Value | |
|---|---|---|---|
| Before procedure | 0.21 ± 0.16 | 0.15 ± 0.05 | 0.11 |
| After procedure | 0.31 ± 0.12 | 0.25 ± 0.11 | 0.10 |
a Values are expressed as mean ± SD.
According to Table 3, in the intervention group receiving aerosolized surfactant, the need for the second dose of surfactant, invasive mechanical ventilation, pneumothorax, bronchopulmonary dysplasia, IVH (grade III or IV), duration of nCPAP, and death were lower than those of the control group receiving surfactant through INSURE method; however, the difference was not significant for any of the aforementioned indicators.
| Variables | INSURE Group | Aerosolized Group | P-Value |
|---|---|---|---|
| Need for second dose of surfactant | 0.771 | ||
| Yes | 10 (40) | 9 (36) | |
| No | 15 (60) | 16 (64) | |
| Invasive mechanical ventilation | 0.145 | ||
| Yes | 7 (28) | 3 (12) | |
| No | 18 (722) | 22 (88) | |
| Pneumothorax | 0.500 | ||
| Yes | 2 (8) | 1 (4) | |
| No | 23 (92) | 24 (96) | |
| Bronchopulmonary dysplasia | 0.269 | ||
| Yes | 6 (24) | 3 (12) | |
| No | 19 (76) | 22 (88) | |
| Intraventricular hemorrhage (grade III or IV) | 0.221 | ||
| Yes | 5 (20) | 2 (8) | |
| No | 20 (80) | 23 (92) | |
| Nasal continuous positive airway pressure duration (Day) | 5.42 ± 4.86 | 3.5 ± 3.27 | 0.089 |
| Death | 2 (8) | 1 (4) | 1.00 |
a Values are expressed as No. (%) or mean ± SD.
5. Discussion
The administration of the surfactant through a noninvasive or less invasive approach has been the subject of several studies performed in the last two decades. In a study performed by Kattwinkel et al. in 2004, 23 neonates within the gestational age range of 23 - 27 weeks weighing within 560 - 1804 g at birth received surfactant. In vaginal delivery, once the neonate’s head appeared in the perineum, the delivery provider prevented the birth of shoulders and provided a timespan for the neonatologist required for administering 3 - 4.5 mL of surfactant (Infasurf) by inserting the tip of a catheter into the posterior pharynx. For neonates born via cesarean section, the procedure was performed when the neonate’s head appeared in the surgical incision area, and then, while the neonate was allowed to complete the birth process, CPAP was applied using a 10-cm water pressure mask. The results showed that 13 out of the 15 neonates born through vaginal delivery and 3 out of the 8 neonates born through cesarean section did not need any additional respiratory support. The rest required nCPAP support (27).
In a study conducted by Berggren et al. in 2000, 32 neonates were involved in the RDS process with 27 - 34 weeks of gestation in two groups, each consisting of 16 cases undergoing CPAP and CPAP in conjunction with surfactant administration using a nebulizer. The aforementioned study did not show statistically significant results regarding the prevalence of the need for invasive mechanical ventilation, patent ductus arteriosus, IVH, air leak, and CLD between the two groups (28).
In another study conducted by Zhang et al. in 2004 on the administration of intra-amniotic surfactant to prevent the occurrence of RDS and its complications, of the 45 pregnant women being exposed to preterm labor, intra-amniotic surfactant was administered for 15 pregnancies, and 30 pregnancies were considered the control group. The occurrence of RDS was significantly higher in the control group (29).
In 2007, a study was carried out by Kribs et al. (18) on the administration of surfactant using a trachea catheter. During a 13-month period, 29 neonates born at University Cologne Children’s Hospital in Germany within 23 - 27 weeks of gestation received nCPAP by Infant Flow Driver (EME, Brighton, UK) after birth. In case FiO2 ≥ 40% was required to maintain SpO2 within the range of 85 - 93%, the neonates were administered surfactant through an endotracheal catheter. For performing this therapeutic intervention, 0.025 mg/kg of atropine is first given to the neonate intravenously, and then the neonate’s head is placed in a position similar to intubation. Afterward, a 4F feeding tube with only one end hole connected to a syringe containing 100 mg/kg of Survanta with a mark 1.5 cm away from the end made by a Magill forceps in the intubation position is inserted into the trachea using a laryngoscope so that the mark on the catheter is at the same level as the vocal cords. The catheter is held in place by the fingers of the right hand, and the laryngoscope is removed. Surfactant is then administered gradually over 1 to 3 minutes. Demonstrations, such as hypoxia, heart rate, coughing, and choking, can occur during the treatment, which need to be managed and can be accompanied by a temporary cessation of the treatment. This group of neonates, along with another group of 34 neonates (control group), with a mean of 25 weeks of gestation who were also managed using the same treatment but whose surfactant was administered through INSURE method, were statistically compared in terms of IVH, PVL, CLD, pulmonary interstitial emphysema (PIE), necrotizing enterocolitis (NEC), and rupture of membrane. The prevalence of IVH (grades III and IV) and PIE in the control group showed a significant increase.
The administration of surfactant by laryngeal mask has also been the focus of a limited number of studies. In a study conducted by Sadeghnia et al. in 2013 administrating surfactant by i-gel in neonates weighing more than 2000 g, the a/APO2 gradient in surfactant administration by i-gel was significantly higher than the same gradient in the administration of surfactant by INSURE (19).
Regarding the administration of surfactant through aerosolization, a study was performed by Finer et al. in 2006 in which 17 newborns with RDS with 29 - 32 weeks of gestation received surfactant by CPAP and nebulizer administered by Lucinactant and Aerosurf. In the aforementioned study, no mortalities were reported, and no neonatal air leakage syndromes and NEC occurred. Thirteen newborns did not need supplemental oxygen at day 28, with four neonates having markers of CLD (21).
In a study performed by Bochenek et al. in 2018, Survanta was administered using a nebulizer to neonates with less than 37 weeks of gestation with RDS. The neonates requiring intubation during the resuscitation process, with congenital anomalies, and with pneumothorax were excluded from this study. A total of 17 newborns with less than 37 weeks of gestation with RDS participated in the study whose mean age was estimated at 35 weeks, 15 of whom underwent a single course of Survanta under noninvasive ventilation (30).
5.1. Conclusions
Studies on surfactant administration with approaches having minimal invasive indicators have been vastly conducted over the last two decades. Although the study by Kattwinkel et al. was conducted with the aim of avoiding trachea intubation, prophylactic surfactant administration was used in this approach, which is in contrast to the early rescue surfactant administration approach. Currently, the prophylactic administration of surfactants cannot be regarded as a strong approach to surfactant administration according to s meta-analysis study performed by Soll and Morley. The same limitation seems to exist in the study conducted by Zhang et al. (2, 27, 29).
The administration of intratracheal surfactant by thin catheter was also considered by researchers, such as Kribs et al. In these studies, catheters can be either rigid or soft, as in the study carried out by Kribs (2016), in which a soft catheter was inserted into the trachea using Magill forceps. However, the use of these catheters requires using a laryngoscope, which still makes this approach invasive (18, 31).
In the study conducted by Sadeghnia et al., surfactant was administered with a supraglottic device approach. The study was performed using i-gel for neonates weighing 2000 g and higher. Although this approach is less invasive since no laryngoscope was used, due to structural limitations in the laryngeal mask, it cannot be used for very low birth weight neonates who are regarded as the target population in RDS treatment (19).
In 2006, a study was conducted by Finer et al. in which 17 RDS newborns with 29 - 32 weeks of gestation were administered Lucinactant with Aerosurf. However, the aforementioned study was not an RCT. Likewise, Bochenek et al. carried out a study in 2018 in which 17 neonates with an average of 35 weeks of gestation with RDS received Survanta using a jet nebulizer, which again was not an RCT (21, 30).
In the present study, Survanta was administered by a mesh nebulizer, and, as shown in Table 2, after receiving surfactant, a/PAO2 gradient showed no significant difference between the two groups. As shown in Table 3, there was no statistically significant difference between the two groups in terms of the side effects under investigation.
In conclusion, it can be mentioned that, although the studied indicators in this study did not show any significant difference, due to the unique noninvasive nature of Survanta administration using a mesh nebulizer, it seems justifiable to conduct further studies since the sample size of the present study was relatively small, which is one of the limitations of this study.
5.2. Limitations
The main limitation the present study suffered from was the limited sample size, which could have had some effects on the significance of the results.