1. Context
Jaundice is one of the most common disorders affecting newborns and one of the major reasons for re-admission during the neonatal period (1). Neonatal jaundice is characterized by the yellowish hyperpigmentation of the skin, mucous membranes, or organs (2). The pathogeny of neonatal jaundice is hyperbilirubinemia (identified as total serum bilirubin (TSB) at least 171 μmol/L for premature neonates or at least 256 μmol/L for term neonates) (2). Neonatal jaundice occurs in up to 60 - 80% of preterm and term neonates and in 10% of breast-feeding neonates (3). Severe hyperbilirubinemia increases the risk of complications or brain disorders in neonates (4). These complications include a wide range of mild-to-severe clinical conditions, such as bilirubin-induced neurologic dysfunction, bilirubin encephalopathy or acute bilirubin encephalopathy, and kernicterus (4). Globally, it is predicted that severe hyperbilirubinemia influences at least 481,000 near-term or term neonates annually, more than 63,000 cases of whom survive with moderate or severe disability (5, 6). Thus, appropriate evaluations and therapeutic interventions to prevent and/or reduce bilirubin-induced neurotoxicity are urgently needed (7).
Several guidelines were provided by the American Academy of Pediatrics for the management of neonatal jaundice (8). Conventional therapies for this disease include standard phototherapy (PT), intensive PT, albumin infusion, and/or exchange transfusion (ET). Currently, PT is a noninvasive, acceptable, safe, and effective treatment (9). However, light exposure to PT might result in water loss, hypocalcemia, circadian rhythms disorder, allergic diseases, or the occurrence of new melanocytic nevi (10). In addition, intensive PT might lead to the photo-oxidation of albumin, resulting in a decrease or complete inhibition of binding affinity for bilirubin (11). The ET is indicated in severe neonatal hyperbilirubinemia when other standard therapeutic strategies, such as PT, have failed and the risk of acute bilirubin encephalopathy is high (12).
Albumin infusion has been used as an adjuvant therapy to PT or prior to ET, improving the outcomes of neonatal hyperbilirubinemia (13). Studies have evaluated the effect of albumin infusion prior to ET with respect to bilirubin removal, the clinical outcomes of which showed variable results. Shahian and Moslehi reported that an infusion of 1 g/kg albumin one hour prior to ET could significantly reduce the post-ET TSB and the duration of PT (14). Nevertheless, Chan and Schiff observed no significant difference in bilirubin removal following albumin infusion prior to ET (15). Given that albumin is a blood product and a scarce and expensive resource, its administration could increase the potential risk of infection and adverse reactions. Albumin infusion might also induce several potential side effects, including fluid overload. However, a systematic review of albumin infusion in preterm neonates revealed no sufficient evidence to consider albumin infusion being associated with significant adverse reactions.
2. Objectives
The present study aimed to assess the efficacy and safety of pre-exchange transfusion (pre-ET) albumin infusion for neonatal hyperbilirubinemia.
3. Methods
3.1. Eligibility Criteria
The study protocol (CRD42018117279) was registered on PROSPERO (https://www.crd.york.ac.uk/prospero/). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to conduct the meta-analysis. Eligibility criteria were based on the PICOS format. The population included neonates within 28 days of birth with hyperbilirubinemia. The intervention involved PT with human albumin infusion prior to ET. The comparator was PT without human albumin infusion prior to ET. The outcomes included post-ET TSB at 6 and 12 hours, the incidence of acute bilirubin encephalopathy, the need for repeating ET, the duration of PT (hour), death prior to hospital discharge, and the incidence of adverse reactions during albumin infusion. Moreover, the study design included randomized controlled trials (RCTs). The diagnostic criteria for neonatal jaundice followed international guidelines (8, 16).
3.2. Search Strategy
Relevant studies from China National Knowledge Infrastructure, Wei Pu Information, Wan Fang Data, Chinese Biomedical Literature Database, EMBASE, Medline, Cochrane Register of Controlled Trials, and ClinicalTrials.gov were explored. The following Medical Subjects and Headings (MeSH) terms and keywords were used:
(1) “Albumins”
(2) “Hyperbilirubinemia” or “Bilirubinemia” or “Jaundice” or “Bilirubin” or “Hematoidin” or “Bilirubinate” or “Icterus”
(3) “Infant” or “Newborn” or “Neonate”
The above-mentioned terms were used separately or combined using the string terms “OR” and “AND”. All published articles from inception to 31st December 2021 were retrieved.
3.3. Screening and Selection of Studies
Two different authors independently screened the articles according to the eligibility criteria listed above. A third author resolved any controversy in the process.
3.4. Data Extraction
The first author's information and publication year, sample size, study design, inclusion/exclusion criteria, criteria for conducting ET, control groups, intervention groups, intervention dosage, gestational age, birth weight, and outcomes were extracted by two different authors independently.
The primary outcomes were post-ET TSB at 6 and 12 hours and the incidence of acute bilirubin encephalopathy. The secondary outcomes included the need for repeating ET, the duration of PT (hour), death prior to hospital discharge, and the incidence of adverse reactions during albumin infusion.
3.5. Quality Assessment Methods
The Cochrane Collaboration’s Risk of Bias (17), including seven items, was applied by two authors to evaluate the quality of the included studies. The quality of the studies was assessed as low risk, high risk, or unclear risk (17). A third author resolved any disagreements by discussion.
3.6. Statistical Analysis
The data were analyzed and compiled using Review Manager software (RevMan version 5.3, The Cochrane Collaboration, 2011). Risk ratio (RR) and 95% confidence interval (CI) were calculated for dichotomous data; nevertheless, mean difference (MD) was used for continuous data. I-squared (I2) statistics were used to perform the heterogeneity tests, and a P-value less than 0.05 was considered statistically significant. For I2 ≥ 75%, significant heterogeneity was considered, and a random effects model (REM) was selected; otherwise, a fixed effects model (FEM) (18) was adopted.
3.7. Publication Bias
When there were more than 10 included studies in a meta-analysis, publication bias was detected using Funnel plots (19).
4. Results
4.1. General Description of Overall Studies
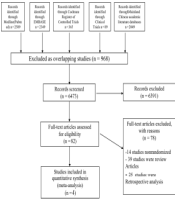
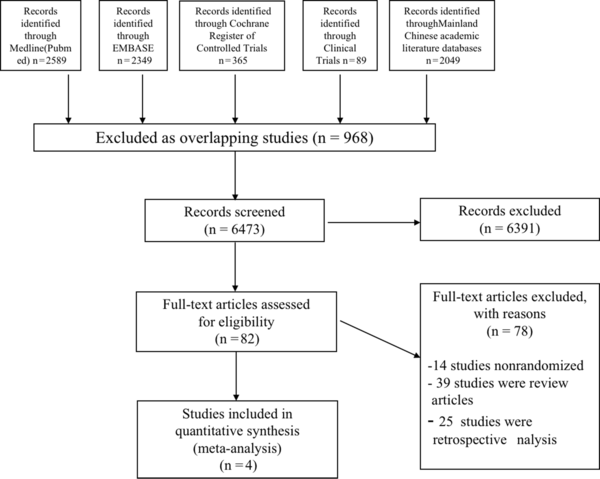
A total of 7441 citations were retrieved using the search strategy described above. After the removal of duplications, 6473 articles remained. After screening the titles and abstracts, 82 full-text articles were retrieved and reassessed for eligibility. Finally, four studies met the inclusion criteria and were included in the systematic review (Figure 1).
The four included trials (13, 14, 20, 21) involved 195 neonatal jaundice participants. All participants in the intervention groups were given intravenous albumin infusion combined with ET; nonetheless, the participants in the control groups were given a placebo combined with ET. During the period of albumin infusion, all the participants in both groups were given PT. In three of the trials (14, 20, 21), the participants were administered 20% human albumin; nevertheless, 5% human albumin was used in only one of the trials (13). In all studies, albumin was used prior to ET at a dosage of 1 g per kilogram body weight. Table 1 shows a summary of the characteristics of the included studies.
| N | Study (First Author, Year) | Sample | GA (Week) a | Birth Weight (g) a | Age on Admission (day) a | Criteria to ET | Intervention Group | Dosage of Albumin | Control Group | Endpoints |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alharis et al., 2020 (21) | Follows AAP guidelines for exchange transfusion | 20% human albumin combined with ET | A dose of 1 g/kg | ET | Post-ET TSB levels at 6 and 12 hours; acute bilirubin encephalopathy; need for repeating ET; duration of PT; incidence of death prior to hospital discharge; adverse reactions during albumin infusion | ||||
| Treatment | 24 | 38.62 ± 0.78 | 3122 ± 296 | 5.6 ± 0.99 | ||||||
| Control | 29 | 38.25 ± 0.8 | 3091 ± 262 | 5.2 ± 0.98 | ||||||
| 2 | Dash et al., 2015 (20) | When two TSB values at least 4 hours apart were 2 mg/dL or more below the PT threshold for that postnatal age | 20% human albumin combined with ET | A dose of 1 g/kg | 0.9% saline at 5 mL/kg combined with ET | Post-ET TSB levels at 6 and 12 hours; acute bilirubin encephalopathy; need for repeating ET; duration of PT; Incidence of death prior to hospital discharge; adverse reactions during albumin infusion | ||||
| Treatment | 23 | 38.2 ± 1.5 | 2952 ± 382 | 4.98 ± 2.47 | ||||||
| Control | 27 | 37.7 ± 1.7 | 2926 ± 626 | 4.46 ± 1.75 | ||||||
| 3 | Mitra et al., 2011 (13) | Defined as the inability to produce a decline of 1 - 2 mg/dL within 4 hours after the initiation of PT | 5% human albumin combined with ET | A dose of 1 g/kg | Intravenous fluid at 20 mL/kg combined with ET | Post-ET UCB levels at 6 and 12 hours; acute bilirubin encephalopathy; need for repeating ET; duration of PT; Incidence of death prior to hospital discharge; adverse reactions during albumin infusion | ||||
| Treatment | 21 | 34.5 ± 1.65 | 1619 ± 324 | 6.23 ± 1.6 | ||||||
| Control | 21 | 34 ± 1.6 | 1660 ± 320 | 6.67 ± 1.43 | ||||||
| 4 | Shahian and Moslehi, 2010 (14) | The inability to produce a decline of 1 to 2 mg/dL within 4 hours after the initiation of PT | 20% human albumin combined with ET | A dose of 1 g/kg | ET | Post-ET TSB levels at 6 and 12 hours; acute bilirubin encephalopathy; need for repeating ET; duration of PT; Incidence of death prior to hospital discharge; adverse reactions during albumin infusion | ||||
| Treatment | 25 | 39.3 ± 1.2 | 3239 ± 585 | 7 ± 1.1 | ||||||
| Control | 25 | 39.5 ± 1.5 | 3264 ± 428 | 8 ± 1.0 |
Description of Characteristics, Baseline, and Outcomes of Four Randomized Controlled Trials Included in This Meta-analysis
4.2. Assessment of Risk of Bias
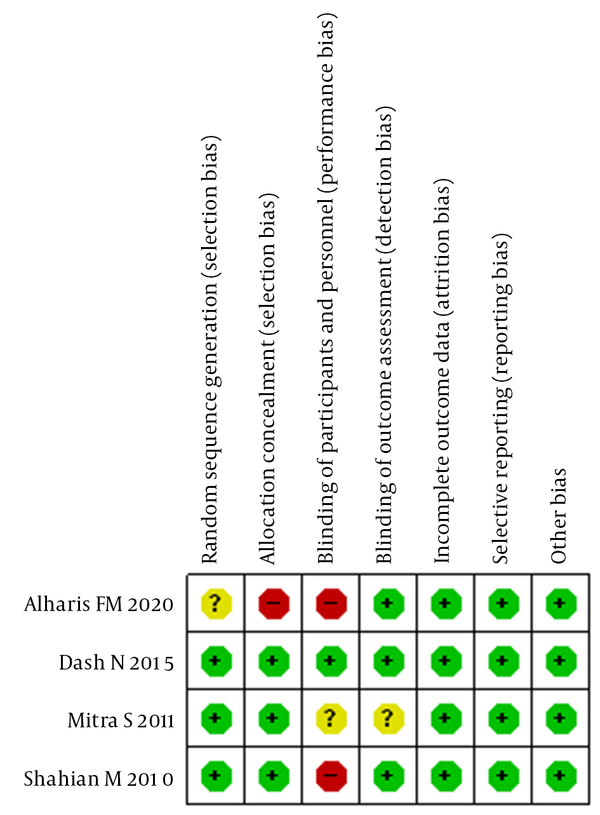
Overall, the quality of the included studies was deemed fair to good (Figure 2). Most included studies specified the sequence generation process and reported allocation concealment. All blinding of participants and personnel was assessed as low risk, except for one trial in which odd numbers were used for the control group and even numbers for the albumin group. The blinding of outcome assessment was also assessed as low risk in all trials, as blinding did not affect the measurement of TSB. All studies were reported with low attrition bias, as none of the participants was lost to follow-up. Reporting bias was assessed as low risk in all studies. None of the studies was observed to be at high risk of for-profit bias. The detailed information is summarized in Table 2.
| Bias | Authors’ Judgment | Support for Judgment |
|---|---|---|
| Shahian and Moslehi, 2010 (14) | ||
| Random sequence generation (selection bias) | Low risk | The random numbers were computer generated. |
| Allocation concealment (selection bias) | Low risk | Slips bearing the allocated group were placed in serially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Twenty-five neonates in the intervention group received intravenous 20% human albumin one hour before exchange; nevertheless, the control group only underwent a blood exchange. |
| Blinding of outcome assessment (detection bias) | Low risk | TSB was measured every 6 hours for both groups during the first 24 hours following the exchange using a Unistat® bilirubinometer. |
| Incomplete outcome data (attrition bias) | Low risk | None |
| Selective reporting (reporting bias) | Low risk | Undiscovered |
| Other types of bias | Low risk | Undiscovered |
| Mitra et al., 2011 (13) | ||
| Random sequence generation (selection bias) | Low risk | Randomization was performed using statistical software. |
| Allocation concealment (selection bias) | Low risk | Slips containing the allocated group were placed in serially numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The infusion of 5% albumin solution was performed for newborns in the intervention group 2 hours before the exchange transfusion. The intravenous maintenance fluid at 20 mL/kg was administered to newborns in the control group for 2 hours. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Two senior pediatricians clinically examined the newborns for evidence of patent ductus arteriosus, necrotizing enterocolitis, and pulmonary edema independently. K-statistics was utilized to evaluate inter-observer variations in evaluating the severity of birth asphyxia and albumin infusion-associated complications. |
| Incomplete outcome data (attrition bias) | Low risk | None |
| Selective reporting (reporting bias) | Low risk | Undiscovered |
| Other types of bias | Low risk | Undiscovered |
| Dash et al., 2015 (20) | ||
| Random sequence generation (selection bias) | Low risk | Randomization into two groups was based on a web-generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in opaque, sealed envelopes which were kept by a person not involved in any other aspect of the study. |
| Blinding of participants and personnel (performance bias) | Low risk | Separate personnel, in a separate room away from the patient care area, prepared the study drug. Blinding was ensured using special black-colored, completely opaque syringes and brown-colored opaque extension intravenous tubing for loading and administration of study drugs. |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators, members of the treatment team, and laboratory technicians remained masked during the intervention. |
| Incomplete outcome data (attrition bias) | Low risk | None |
| Selective reporting (reporting bias) | Low risk | Undiscovered |
| Other types of bias | Low risk | Undiscovered |
| Alharis et al., 2020 (21) | ||
| Random sequence generation (selection bias) | Unclear risk | Randomization was performed by taking the odd numbers as the control group and even numbers as the albumin group. |
| Allocation concealment (selection bias) | High risk | Taking the odd numbers as the control group and even numbers as the albumin group |
| Blinding of participants and personnel (performance bias) | High risk | Taking the odd numbers as the control group and even numbers as the albumin group |
| Blinding of outcome assessment (detection bias) | Low risk | TSB was measured by drawing blood using heparinized capillary tubes by pricking the heal and then centrifuged with serial number 0000526-01.00 with 1000 rounds per minute and then placed in a bilirubinometer. |
| Incomplete outcome data (attrition bias) | Low risk | None |
| Selective reporting (reporting bias) | Low risk | Undiscovered |
| Other types of bias | Low risk | Undiscovered |
Risk of Bias for Included Studies
4.3. Effects of Intervention
4.3.1. Post-exchange Transfusion Total Serum Bilirubin Levels at 6 and 12 Hours
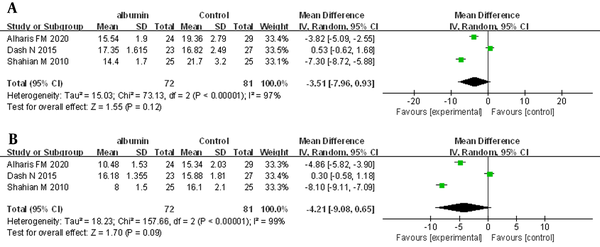
Three trials, in which 153 neonates were enrolled, provided information on post-ET TSB levels at 6 and 12 hours. The REM was used for the primary analysis. The meta-analysis showed no statistically significant difference for post-ET TSB at 6 hours (MD = -3.51 mg/dL, 95% CI: -7.93 - 0.93, I2 = 97%; REM) (Figure 3A). For post-exchange TSB at 12 hours, there was also no statistically significant difference (MD = -4.21 mg/dL, 95% CI: -9.08 - 0.65, I2 = 99%; REM) (Figure 3B).
4.3.2. Acute Bilirubin Encephalopathy
No patients developed acute bilirubin encephalopathy in any of the trials.
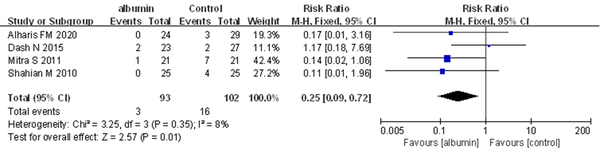
4.3.3. Need for Repeating Exchange Transfusion
Four trials, in which 195 neonates were enrolled, provided information on the neonates who required repeating ET. In the meta-analysis, a statistically significant difference was observed between the two groups for this outcome. Pre-exchange transfusion albumin infusion was observed to be superior to no albumin infusion for reducing the need for repeating ET (RR = 0.25, 95% CI: 0.09 - 0.72, I2 = 8%; FEM) (Figure 4).
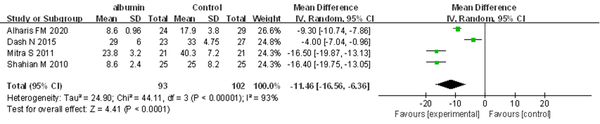
4.3.4. Duration of Phototherapy (Hour)
All trials reported the duration of PT. The meta-analysis revealed that pre-ET albumin infusion was associated with a shorter duration of PT (MD = -11.46 hours, 95% CI: -16.56 to -6.36, I2 = 93%; REM) (Figure 5).
4.3.5. Incidence of Death Prior to Hospital Discharge
No death prior to hospital discharge was reported in any of the trials.
4.3.6. Adverse Reactions During Albumin Infusion
Four studies addressed adverse events, and no adverse effects were observed in the intervention groups.
5. Discussion
5.1. Summary of Main Findings
The study focused on assessing the safety and efficacy of pre-ET albumin infusion for the treatment of neonatal hyperbilirubinemia. Four RCTs were included, most of which were evaluated with a low risk of bias. Only three studies reported the primary outcome of post-ET TSB at 6 and 12 hours. All the included trials focused on the effects of pre-ET albumin infusion on the need for repeating ET and PT duration. The present meta-analysis demonstrated that pre-ET albumin infusion could reduce the need for repeating ET and shorten the duration of PT; however, there was no statistically significant difference in post-ET TSB at 6 and 12 hours between the two groups.
Severe hyperbilirubinemia can lead to brain damage, which is caused by unconjugated bilirubin (UCB) (8). The small amount of UCB, which is not bound to albumin, can pass the blood-brain barrier and disrupt several essential cellular functions, inducing apoptosis, necrosis, kernicterus, or death (22, 23). Therefore, early detection and treatment are important in the prevention of bilirubin-induced encephalopathy. At physiological pH, UCB is poorly water-soluble and needs to be metabolized, which includes being bound to albumin as a dianion at a primary binding site in the liver and then excreted from the body. Furthermore, Wood et al. showed that intensive PT for severe hyperbilirubinemia could cause the photo-oxidation of albumin, thereby leading to the decrease or complete inhibition of its binding affinity for UCB (11). More binding sites need to be provided for UCB to reduce UCB levels, which ultimately decreases the risk of central nervous system toxicity. Therefore, in theory, it is possible to avoid UCB accumulation in the brain by increasing bilirubin-binding capacity within the intravascular compartment by albumin infusion. The current meta-analysis also demonstrated that pre-ET albumin infusion can reduce the need for repeating ET and shorten the duration of PT, thereby validating the efficacy of pre-ET albumin infusion for neonatal hyperbilirubinemia.
Furthermore, the level of UCB in plasma can be decreased by albumin within the intravascular space, allowing unbound tissue bilirubin to move into the plasma to establish equilibration between plasma bilirubin and the extravascular space (24, 25). Albumin infusion prior to ET resulted in more efficient removal of intravascular UCB, a consequent reduction in TSB, and a lower rebound increase in post-ET UCB (26). Nevertheless, the post-ET TSB levels at 6 and 12 hours of the intervention group were not significantly different from those of the control group. Why did the post-ET TSB at 6 and 12 hours not decrease after pre-ET albumin infusion? It is likely that the level of post-ET TSB depends on both pre-ET extravascular and vascular bilirubin levels and that the relationship between TSB and extravascular bilirubin levels was obscured due to significant variations in vascular bilirubin-albumin interactions (27). As a result, despite similar pre-ET UCB in two groups, there could be variations in extravascular bilirubin levels, eventually affecting the final outcome (28). In addition, albumin infusion for neonatal hyperbilirubinemia might have increased the non-binding fraction of serum albumin; nevertheless, the ratio of bilirubin to albumin might have remained the same (29). Therefore, it follows that an estimation of UCB is important, and the assessment of the level of UCB might be more appropriate for evaluating the efficacy of pre-ET albumin infusion in neonatal hyperbilirubinemia.
On the other hand, three of all the included trials provided information on post-ET TSB levels at 6 and 12 hours. Only one trial showed that the administration of 1 g/kg of 20% albumin prior to ET is not superior to 0.9% saline in reducing post-ET TSB levels at 6 and 12 hours (20). Notably, the aforementioned study (20) had a lower baseline serum albumin level than two other studies (14, 21) (2.8 vs. 3.2 - 3.4 g/dL). It was possible that 1 g/kg of 20% albumin was not enough to provide sufficient binding sites for bilirubin due to a lower baseline serum albumin level, leading to not observing an increase in bilirubin removal following ET. Earlier studies with a positive effect of albumin infusion had higher baseline serum albumin levels (3 - 3.5 g/dL), and albumin levels were usually at least 5 g/dL after infusion (14, 15, 26, 30). A higher baseline serum albumin level could provide more binding sites for bilirubin and might facilitate the diffusion of bilirubin into intravascular space better. Whether the dose of albumin priming needs to be determined according to baseline serum albumin levels or whether larger doses of albumin for those neonates with low baseline serum albumin levels need to be used should be evaluated. Therefore, the results about not observing an increase in bilirubin removal following ET need more well-designed large RCTs to be revalidated.
5.2. Strengths and Limitations
The current study has been the first meta-analysis to show that pre-ET albumin infusion can reduce the need for repeating ET and shorten the duration of PT; however, there was no statistically significant difference between the two groups with respect to post-ET TSB at 6 and 12 hours. The present study included a very limited number of only four relevant RCTs. Therefore, more RCTs with larger population sizes are urgently needed to validate the efficacy of pre-ET albumin infusion for neonatal hyperbilirubinemia. In addition, this meta-analysis demonstrated that pre-ET albumin infusion could shorten the duration of PT; nevertheless, very high heterogeneity was observed among studies (I2 = 93%). This might be due to the differences in the production of albumin, types of PT, or the manufacture of used bilirubinometer.
Furthermore, an important limitation might be a history of intravenous fluid supplementation in most study subjects before the intervention. Intravenous fluid supplementation could result in a faster drop in TSB levels due to its effects on volume expansion and glomerular filtration rate (31, 32). The effect of intravenous fluid supplementation on bilirubin-albumin binding and the movement of bilirubin across capillaries remains unclear. Some studies report an increase in the removal of bilirubin after infusion with 1 g/kg of albumin 1 - 4 hours prior to ET (13, 14, 33); however, other studies were unable to demonstrate similar effects (15, 30, 34). In addition, albumin infusion leads to volume expansion, which could also independently cause a reduction of bilirubin level after administration (25). To confirm the hypothesis that albumin infusion might enhance the removal of bilirubin through bilirubin binding, the confounding effect of the volume expansion of albumin infusion has to be counterbalanced by using a mother fluid. Most of the included studies did not use any mother fluid. Therefore, further well-designed large RCTs are needed to assess the efficacy of albumin infusion.
5.3. Implications for Further Studies
Based on the present meta-analysis, pre-ET albumin infusion might result in short-term benefits, including reducing the need for repeating ET and shortening the duration of PT. However, all of the included studies were not large-scale, multi-centered trials. It is suggested to conduct future studies to assess the aforementioned outcomes, especially post-ET TSB levels. Moreover, whether to use larger doses of albumin for those neonates with low baseline serum albumin levels needs to be evaluated. The efficacy and safety of pre-ET albumin infusion need to be further elucidated.
5.4. Conclusions
Based on four studied trials, pre-ET albumin infusion appears to be safe and effective in reducing the need for repeating ET and shortening the duration of PT. However, due to limitations of the number and types of studies, there is a need for further well-designed, large RCTs to explore the efficacy and safety of pre-ET albumin infusion for neonates with hyperbilirubinemia before being able to make a conclusive recommendation about pre-ET albumin infusion for the treatment of neonatal hyperbilirubinemia.