1. Background
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized by insulin deficiency and hyperglycemia (1). In 2021, around 8.4 million people worldwide had T1DM, and it is estimated that this number will reach 17.4 million by 2040, doubling in the next 20 years (2). Diabetes mellitus (DM) causes microvascular damage in multiple organs (3), which can lead to visual loss in adults. Other manifestations of DM include cataracts, refractive errors, macular edema, retinopathy, optic nerve damage, and ocular muscle dysfunction (4-6). Patients with uncontrolled diabetes are also at a higher risk of developing ocular complications such as dry eye syndrome, keratopathy, increased epithelial growth after Laser-Assisted In-Situ Keratomileusis surgery (LASIK), contact lens complications, iris neovascularization, vitreous depigmentation, and bleeding (7, 8).
Visual system impairment from childhood can severely diminish the quality of life while imposing high economic and emotional costs on society (9). The incidence and causes of ocular complications of T1DM in adults have been well described, but information is lacking on both the prevalence and types of complications among children and adolescents (10-12).
2. Objectives
The aim of this study was to evaluate the prevalence of ocular complications (especially those affecting the anterior segment) in children and adolescents with T1DM and to assess the relationship of these complications with age, gender, duration of T1DM, and hemoglobin A1C (HbA1c).
3. Methods
3.1. Study Design
This cross-sectional study was conducted through the collaboration between the Pediatrics Department of Ali-Ibn-Abitaleb Hospital, Ali Asghar Pediatric Hospital, and Al-Zahra Ophthalmology Hospital, all affiliated with Zahedan University of Medical Sciences in Zahedan, Iran. The patient selection was done by convenient sampling, and screening was done by a pediatrics endocrinologist, who then referred the patients to a corneal and anterior segment ophthalmologist for further examination.
The inclusion criteria were: ({Katsarou, 2017 #1}1) a type 1 diabetes mellitus diagnosis based on both the World Health Organization (WHO) and International Society for Pediatrics and Adolescent Diabetes (ISPAD) criteria (13, 14); (2) age under 18 years; and (3) consent and cooperation in all the examinations related to this study. The exclusion criteria included a history of ocular disease, surgery, or trauma. Based on the results of Geloneck et al. (15), the sample size was determined to be 76 patients (P: 19.5%, ɑ: 90%, Z-Score: 1.65, ß: 7.5%).
3.2. Data Collection
Patient information, including date of birth, gender, duration of T1DM, past medical history, and medication history (insulin regimens such as Lantus® -Lantus Solostar Injection, Sanofioaventis, Germany- Aspart® -Novorapid Flexpen, Novo Nordisk, Brazil-, Levemir® -Levemir injection, Novonordisk, Denmark-, NPH insulin -Lansulin N Vial, Exir Pharmaceutical Co., Iran- and Regular insulin-Lansulin R Vial, Exir Pharmaceutical Co., Iran-), was recorded on Form A. Baseline glycated hemoglobin levels (HbA1c) were measured via high-performance liquid chromatography.
Subsequently, patients were examined by a corneal specialist at Al-Zahra Zahedan Ophthalmology hospital, Zahedan, Iran. Visual acuity (VA) was tested using a Snellen chart (MEDIZS LC13, MEDIZS, South Korea). Tropicamide ophthalmic drops (MYDRAX® 1%, Sina Darou, Iran) were applied to both eyes every 5 minutes for three consecutive times. Optometry examinations were performed 20 minutes after pupillary dilation. The eyelid, conjunctiva, cornea, anterior chamber, iris, pupil, and lens of each eye were examined using a slit-lamp biomicroscope (HAAG-STREIT BM900, HAAG-STREIT, Switzerland), and the obtained data were recorded on Form B by the ophthalmologist. For higher accuracy, the ophthalmologist was blinded to the information recorded on Form A.
3.3. Statistical Analysis
We used descriptive statistics to analyze the demographic characteristics of the study. Confounding adjustments were performed using multivariate linear regression to assess whether demographic or clinical factors directly influenced the results. The distributions of quantitative data were assessed for normality using the Kolmogorov-Smirnov Test. Continuous and categorical variables were analyzed using the t-test and chi-squared test, respectively. Confidence intervals were computed at the P-value of 0.05 level, and analyses were performed using SPSS Statistics version 26 (IBM Corporation, Chicago, IL, USA).
3.4. Ethical Considerations
This study was conducted in adherence to the tenets of the Declaration of Helsinki and was ethically approved by the Ethics Committee of Zahedan University of Medical Sciences (Approval ID: IR.ZAUMS.REC.1401.097). Informed consent was obtained from the children and their parents.
4. Results
A total of 76 T1DM children were selected and evaluated for ocular complications. The average age was 12.04 ± 3.70 years (range: 4 - 20 years), with 39 (51.3%) boys and 37 (48.7%) girls. The duration of T1DM ranged from 1 to 11 years, with an average of 4.69 ± 2.49 years. Fifty-nine patients (77.6%) had no other remarkable medical histories, but the frequencies of other diseases were as follows: 5 patients with hypothyroidism (6.5%), 5 patients with celiac disease (6.5%), and 2 with concomitant celiac and liver diseases (2.6%). Other conditions found included liver disorders, PCOS (polycystic ovarian syndrome), growth disorders, polyneuropathy, and the concomitant occurrence of celiac disease with hypothyroidism, each occurring in 1 patient (1.3%). The average HbA1c levels were 10.24 ± 2.47% (range: 4.5 - 17). The insulin regimens were as follows: Lantus+Aspart (60 participants, 79%), Levemir+Aspart (11 participants, 14.5%), and NPH+regular (5 participants, 6.5%). Regarding the stage of puberty, 35 patients (46%) were in the pre-pubertal stage, 32 patients (42.2%) were in the pubertal stage, and there were no patients in the post-pubertal stage (puberty stage was unknown in 9 patients) (Table 1).
| Variables | Values |
|---|---|
| Age | 12.04 ± 3.70 |
| Gender (male, female) | 39, 37 (51.3, 48.7) |
| Duration of T1DM | 4.69 ± 2.49 |
| Insulin regimen: Lantus+Aspart, Levemir+Aspart, NPH+Regular, Missed | 60, 11, 3, 2 (79, 14.5, 3.9, 2.6) |
| Puberty stage: Pre-pubertal, pubertal, post-pubertal, missed | 35, 32, 0, 9 (46, 42.2, 0, 11.8) |
| HbA1c | 10.24 ± 2.47 |
| Past medical history: Celiac disease, hypothyroidism, hepatic disease, PCOS, growth disorder | 8, 6, 3, 1, 1 (10.5, 7.8, 3.9, 1.3, 1.3) |
a Values are expressed as No. (%) or mean ± SD.
Table 2 shows the visual acuity status (evaluated by Snellen chart) in T1DM patients. We found that approximately 85% of our diabetic patients had no refractive errors.
| VA | OD | OS |
|---|---|---|
| Normal vision [VA 6/12 or better (snellen equivalent)] | 63 (82.9) | 60 (78.9) |
| Mild visual impairment (VA worse than 6/12 to 6/18 (snellen equivalent)) | 5 (6.6) | 7 (9.2) |
| Moderate visual impairment (VA worse than 6/18 to 6/60 (Snellen Equivalent)) | 0 (0) | 1 (1.4) |
| Severe visual Impairment (VA worse than 6/60 to 3/60 (Snellen Equivalent)) | 1 (1.4) | 1 (1.4) |
| Blindness (VA worse than 3/60 (Snellen Equivalent)) | 0 (0) | 0 (0) |
| Not cooperative | 7 (9.2) | 7 (9.2) |
| All patients | 76 (100) | 76 (100) |
a Values are expressed as No. (%).
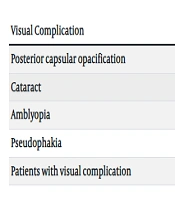
The total prevalence of all visual complications in T1DM was 27 patients (35.5%). The most common visual complication was posterior capsular opacification, affecting 19 patients (24.9%). This was further categorized into multiple dot-shaped opacities in 12 patients (15.7%) and linear haziness opacities in 7 patients (9.2%). Notably, 1 patient (1.4%) with severe visual impairment (VA: 1.10) was diagnosed with a clinically significant cataract. Additionally, the prevalence of amblyopia and pseudophakia were 2.7% and 1.4%, respectively (Table 3).
| Visual Complication | No. (%) |
|---|---|
| Clinically non-significant opacity | |
| Multiple dot shape opacity | 12 (15.7) |
| Linear haziness opacity | 7 (9.2) |
| (Clinically significant opacity) cataract | 1 (1.4) |
| Refractive errors | 8 (10.5) |
| Macular edema | 0 (0) |
| Meibomian gland dysfunction (MGD) | 0 (0) |
| Amblyopia | 2 (2.7) |
| Neuromuscular dysfunction | 0 (0) |
| Pseudophakia | 1 (1.4) |
| Diabetic retinopathy (DR) | 0 (0) |
| Patients with visual complication | 27 (35.5) |
Our results indicate that the incidence of posterior capsule opacification and overall visual complications were significantly related to age (P = 0.022 and P = 0.03, respectively) (Table 4).
a Values are expressed as mean ± SD.
b Independent samples t-test.
There was no significant relationship between visual complications and gender (P > 0.05) (Table 5).
a Values are expressed as No. (%).
b Chi-square test.
c Fisher’s exact test.
In addition, posterior capsule opacification was significantly associated with the duration of T1DM (P < 0.001) (Table 6).
| Visual Complication | Duration on T1DM (Median, 95% CI) | P-Value a | |
|---|---|---|---|
| Yes | No | ||
| Posterior capsular opacification | 8 (6.7 - 9.9) | 4 (3.8 - 4.9) | < 0.001 |
| Cataract | 9 | 4 (3.8 - 4.9) | 0.158 |
| Amblyopia | 3 (1.5 - 8.8) | 4.7 (4.1 - 5.3) | 0.289 |
| Pseudophakia | 4 | 4 (4.1 - 4.6) | 0.974 |
| Patients with visual complication | 5 (4.2 - 6.3) | 4 (3.7 - 5.1) | 0.111 |
a Mann-Whitney test.
There was no significant correlation between visual complications and HbA1c levels; however, a single evaluation of HbA1c is not a reliable test (P > 0.05) (Table 7).
| Visual Complication | HbA1c (Median, 95% CI) | P-Value a | |
|---|---|---|---|
| Yes | No | ||
| Posterior capsular opacification | 10.1 (8.1 - 13.2) | 9.9 (9.6 - 10.8) | 0.615 |
| Cataract | 13.9 | 9.9 (9.7 - 10.8) | 0.684 |
| Amblyopia | 8.9 (6.4 - 12.2) | 9.9 (9.7 - 10.9) | 0.412 |
| Pseudophakia | 9.4 | 9.9 (9.7 - 10.8) | 0.737 |
| Patients with visual complication | 9.9 (9.6 - 11) | 9.6 (9.1 - 11.2) | 0.46 |
a Mann-Whitney test.
5. Discussion
Diabetes mellitus (DM) has several ocular complications involving both the anterior and posterior segments of the eye, including cataracts, corneal pathologies, and retinopathies. Epidemiological studies have found that early detection of diabetic retinopathy (DR) is key to preventing vision loss; therefore, routine screening for DR is necessary (16). Screening guidelines state that the initial eye exam in T1DM patients should occur 5 years after diagnosis and then annually if no DR is found (17, 18). However, our study showed that ocular complications in T1DM are not exclusive to DR, and other complications also require early detection.
Šimunović et al. investigated cataracts as either the first sign of T1DM or one that occurs within 6 months of T1DM diagnosis. Furthermore, the prevalence of early diabetic cataracts varies between 0.7 - 3.4% in children and adolescents with T1DM (10). Essuman et al. reported the prevalence of anterior segment complications such as blepharitis, tear film instability, and cataracts in T1DM to be 79.3%, 65.5%, and 72%, respectively (19). Lu et al. also stated that the risk of cataract in T1DM patients compared to healthy individuals was about 5.8 times higher (20). Therefore, similar to the results of our study, signs of new onset cataracts, like lens opacity, are considered serious early complications of T1DM.
The mechanism of T1DM ocular complications is not well understood; however, prolonged hyperglycemia with ketoacidosis and subsequent dehydration, genetics, nutritional habits, and the environment play important roles in the development of early diabetic cataracts (21-23). The pathophysiology of T1DM involves osmotic damage, oxidative stress, and activation of other metabolic pathways (21-23). Additionally, the prevalence of lens opacity is higher in T1DM (10), which may be related to environmental factors such as higher temperatures and exposure to sunlight, as well as dehydration. Therefore, delayed diagnosis of type 1 diabetes in children and adolescents in the environmental conditions of southeastern Iran (and other similar climates) may cause considerable lens damage.
The health implications of addressing the risk of cataracts in T1DM are significant. Delayed diagnosis can lead to more complications, and anterior segment complications seem more prevalent in youth. We recommend regular ophthalmology visits to determine the occurrence of lens opacification. Additionally, children under 8 years old, who have not yet achieved visual maturity, need to be screened to avoid amblyopia (24).
We found no DR, which may be due to the fact that most of our patients were in the pre-pubertal and pubertal stages. However, in a large cohort of 370 children with both type 1 and type 2 diabetes mellitus, no cases of DR were reported either (15). Thus, detecting DR in the young population is a rare occurrence. Ocular changes associated with T1DM were those related to the thickness of retinal nerve fiber layer, ganglion cell layer, and choroidal stroma occurs in pediatric patients with T1DM (25-27). Wang et al. compared the incidence and risk factors for developing diabetic retinopathy in the youths with type 1 or type 2 diabetes (T2DM) showing that the prevalence of DR in T1DM and T2DM patients were 20.1% and 7.2%, respectively. In addition, T1DM patients developed DR faster than those with T2DM (28).
Our results showed that the prevalence of visual impairment (VI) among diabetic patients was approximately 10% and VI was mild in most cases. Klein et al. studied the 25-year incidence of VI in T1DM patients before the age of 30. They showed that the incidence of VI in T1DM was 13% and increased risk was associated with severe retinopathy, presence of cataracts, and elevated glycosylated hemoglobin levels. They concluded that VI might be reduced by better glycemic and blood pressure control (29). Yet despite our lower mean of age (11.9 versus 24.9 years old), the occurrence of refractive errors in our population were similar, which directs us towards an existing correlation. Shadhan et al. studied the ocular complications in 150 children and adolescents with T1DM. Their results indicate that 16% of patients had ocular complication (almost two-thirds of the cases were cataract and the rest were DR). In addition, all of those who had developed ocular complications were above 10 years old, occurring significantly more in those with a higher duration of T1DM. Among 24 patients with eye complication, 18 patients (75%) had limited joint mobility, 17 patients (70.8%) had HbA1c level more than 10%, 16 patients (66.7%) had short stature, 8 patients had macro-albuminuria (33.3%), and 7 patients (29.1%) had celiac disease (CeD) (30). Interestingly, the simultaneous presence of other autoimmune diseases (such as rheumatic disease, CeD, and hypothyroidism) with T1DM brings attention to their mutual autoimmune origin. Roher et al. did a multicenter longitudinal analysis of 56,514 patients from the German-Austrian study in which they found that the risk of retinopathy and nephropathy were higher in patients with diabetes and CeD compared to those without CeD (31). Therefore, based on the prevalence of CeD and T1DM in our study, as well as the high prevalence of CeD in the southeastern Iran (32), screening for ocular complications in this population is necessary.
The limited population enrolled in the study as well as the lack of a control group limited the generalizability of our results; however, the similar statistical reports to other studies might mitigate these limitations and let the results speak for themselves. Additionally, the cross-sectional nature of the study design -while limiting follow-ups- establishes a pre-existing correlation between early ocular complications and T1DM in pediatrics.
5.1. Conclusions
In conclusion, with a rate of 35.5% ocular complications in T1DM patients, the most prevalent of which is posterior capsule opacification, we suggest routine screening of the anterior segment of the eye for T1DM pediatric patients prior to the presentation of blurry vision or visual acuity decline.