1. Introduction
Arrhythmogenic right ventricular dysplasia (ARVD), a cardiomyopathy characterized by fibrofatty degeneration of the myocardium with progressive dysfunction, electrical instability, and sudden death, occurs in approximately 1 in 5000 people in the United States. Male sex may be associated with higher disease penetrance (1). ARVD occurs as an isolated cardiomyopathy or in the context of two related disorders, Naxos or Carvajal syndrome, which prominently manifest in skin (palmar-plantar keratosis), hair (woolly), and either predominantly the right ventricle (Naxos) or left ventricle (Carvajal) (2).
Naxos disease is an autosomal recessively inherited familial syndrome that was first reported in the Hellenic island of Naxos (3, 4). Cases also have been reported in other Hellenic islands, as well as in Turkey, Israel and Saudi Arabia (4). Carvajal syndrome, as a variant of Naxos disease, has been described in families from India and Ecuador. As mentioned above, clinically it presents with the same cutaneous phenotype and predominantly left ventricle involvement (5).
A 2-bp deletion in the plakoglobin gene has been identified as the cause of Naxos disease. In Carvajal syndrome, 2 different mutations of the desmoplakin gene have been found as causative genes (6). These mutations in genes encoding desmosomal proteins cause defects in the linking sites of these proteins and consequently can interrupt the contiguous chain of cell adhesion, particularly under conditions of increased mechanical stress or stretch, leading to cell death, progressive loss of myocardium, and fibrofatty replacement (5).
The affected members of both syndromes have woolly hair at birth, whereas palmoplantar keratoderma appears during the first year of life, when infants start using their extremities (6). In Naxos disease, the cardiomyopathy clinically manifests in adolescence. Patients may develop progressive heart disease involving the right or both ventricles. Symptoms of right heart failure are found in the final stages when the right or both ventricles are severely affected (3). In Carvajal syndrome, on the other hand, heart disease becomes clinically apparent earlier during childhood as dilated cardiomyopathy. Fifty percent of affected patients develop heart failure, and most of them die during adolescence (5).
In Naxos disease, cardiac histology reveals the characteristic loss of the right ventricular myocardium with fibrofatty replacement. Cardiac histology of Carvajal disease shows areas of extensive myocardial loss and replacement with fibrosis that is very similar to arrhythmogenic right ventricular dysplasia/cardiomyopathy pathology but without the fatty component (7).
2. Case Presentation
We present a nine-year-old girl complaining of dyspnea, easy fatigability and skin lesions. She had a history of an occasional epistaxis and weakness since 20 days before her admission, accompanied by the symptoms and signs of common cold, specially cough, during the last two days.
In the past medical history, the skin disease had started when she was around 4 months old with eruption of some desquamative, crusted and non-pruritic lesions on the plantar surfaces of both lower limbs. After being visited by a dermatologist, since there were similar lesions diagnosed in her two cousins, she was told of the genetic transmission of the rash and that nothing needed to be done. Up to the age of 8 years of age, she also complained of occasional episodes of fatigue for which correction of anemia was suggested.
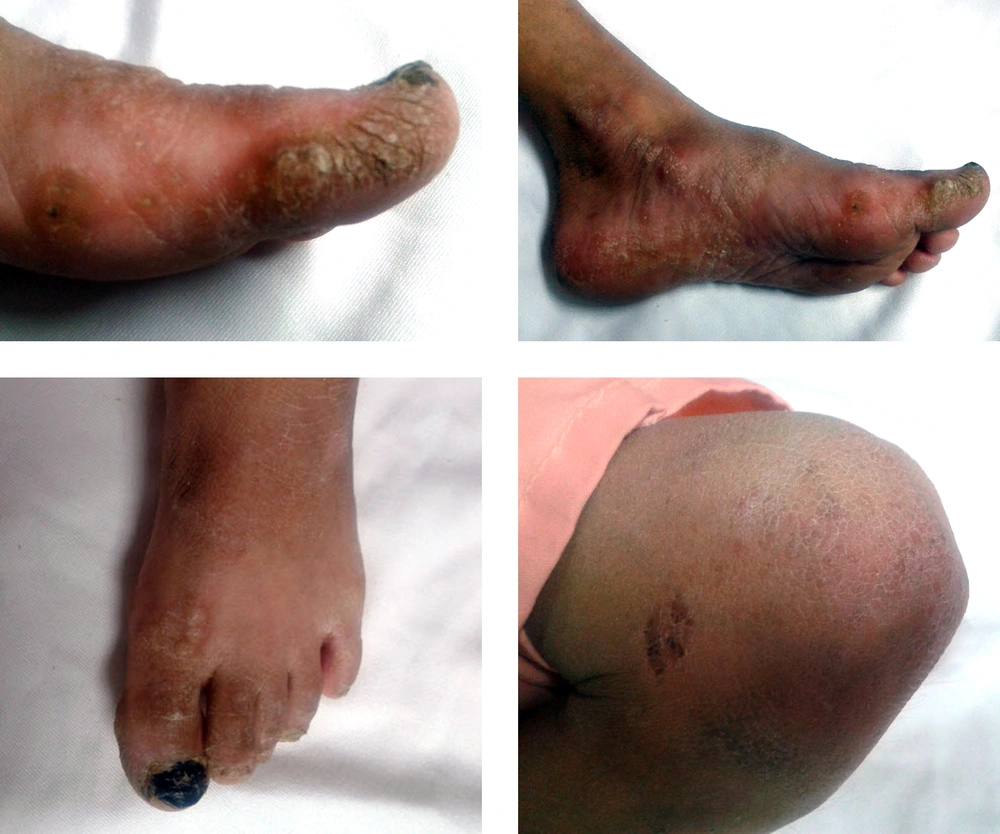
On physical examination, she had respiratory distress and widely spread skin lesions. Vital signs showed tachypnea, tachycardia, normal blood pressure and temperature. The lesions were on the trunk, fingers, toes, and specially on palmoplantar areas. They were crusted with yellowish color, peeling and not bleeding when scratched on (Figure 1). Also the lips seemed dry, brittle and bleeding easily on gentle palpation.
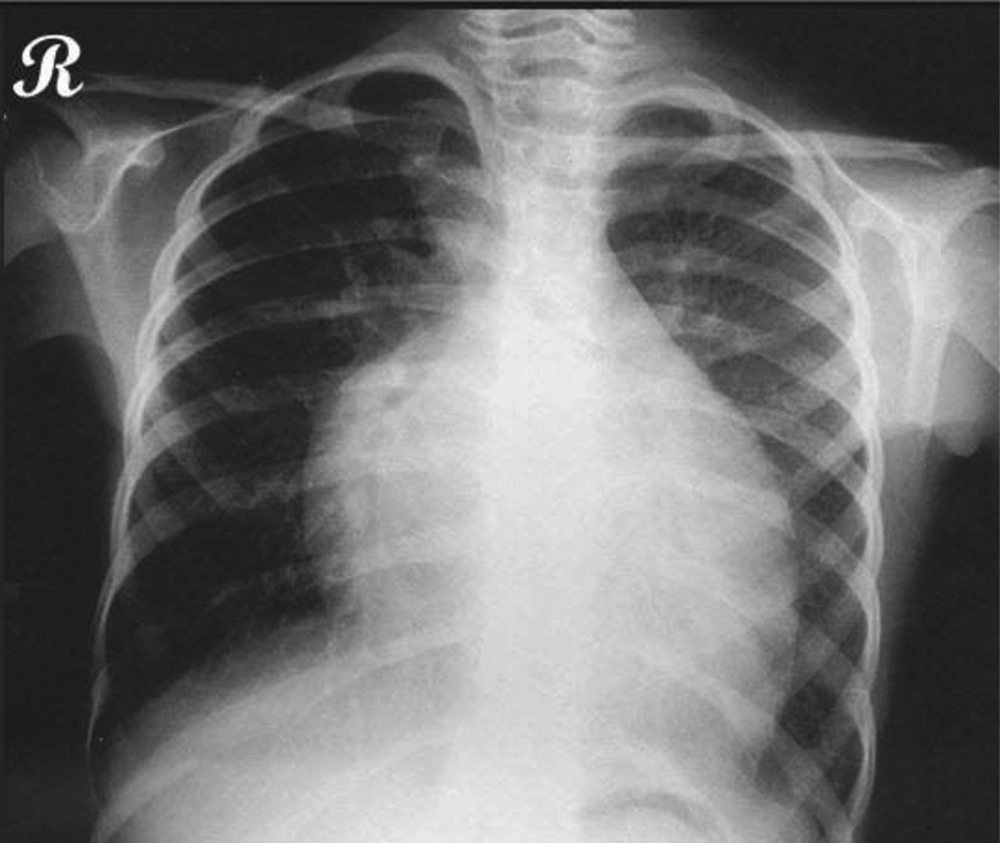
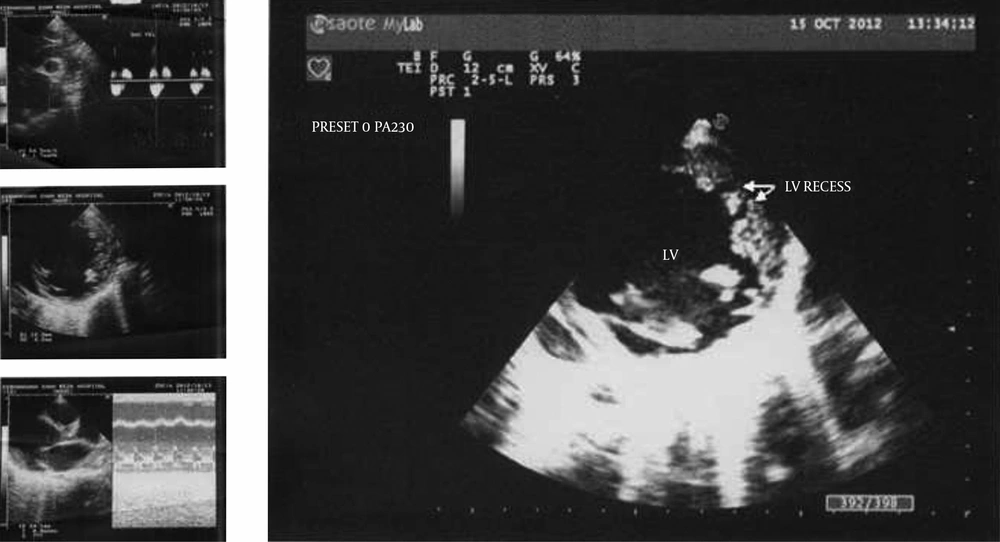
A holosystolic murmur was heard at the apex radiating to the axillary region, and a gallop rhythm was detected. She had no organomegaly except for palpating the liver about 3 to 4 centimeters below the rib edge. No clubbing or more important findings were found. The chest X-ray showed cardiomegaly (Figure 2). On echocardiography, the left ventricle was dilated and had a non-compaction pattern with its ratio to the compacted compartment more than 1.4/1. The M-mode displayed a decreased ejection fraction and an increased E point septal separation (EPSS). Also the color doppler mode showed mild to moderate mitral regurgitation (Figure 3). The patient was admitted to PICU and the monitoring was started. She was put on humidified oxygen and 2/3 maintenance fluid. The lab tests were sent and parenteral medications including dopamine, dobutamine and furosemide begun. Complete blood count showed mild anemia and biochemistry was normal except for the slightly increased liver enzymes, most probably due to hepatic congestion. The dermatological consultation confirmed the diagnosis of Carvajal Syndrome. The patient had a smooth course in the PICU and was discharged a few days afterwards with a good general condition receiving digoxin, furosemide, captopril, sprinolactone and coenzyme Q.
3. Discussion
Cardiac dilation and decreased systolic function, the uniform diagnostic features of dilated cardiomyopathy (DCM), fail to reflect the many causes of DCM. Infectious, metabolic, ischemic, toxic, and hereditary factors have been implicated in the disease pathogenesis (8-12). DCM, with a prevalence of 36.5 per 100,000 population (8), is thus a final common pathway for diverse disease processes that lead to heart failure. Despite a broad differential diagnosis, however, the primary cause of sporadic and hereditary DCM in children is unknown in over 70% of cases (11, 12), and the preclinical cascade of molecular and cellular events leading to heart failure remain poorly understood. Most patients with idiopathic DCM have clinically silent disease in childhood, developing symptoms only in middle age (mean age at diagnosis 45 ± 17 years) (13). The delayed onset of clinically apparent disease suggests a subtle congenital defect in myocardial function inciting a gradual degenerative process that progresses over several decades.
LVNC, a cardiomyopathy of unknown cause results in elements of both left ventricular dilatation and restriction. The condition may be diagnosed at any age, from infancy to young adulthood, and the severity of heart failure varies. The echocardiogram is diagnostic and shows a specific pattern of left ventricular hypertrophy with deep muscular crypts. Patients may be at risk for ventricular arrhythmias and sudden death, as well as mural thromboses and stroke. Although some patients may remain stable for years, others may deteriorate rapidly. Cardiac transplantation has been used successfully in this group of patients (14).
It seems the presence of the isolated non-compaction of left ventricle in the context of Carvajal syndrome has rarely been reported. Because of the paucity of the cases ever diagnosed, it is still too early to get to a definite conclusion about the risk of sudden death and other probable complications of this very special entity. The co-existence of the skin lesions and cardiac involvement in this syndrome, probably proves a common genetic background which in turn needs more investigations so as to be clarified.
The girl whom we report, has been once hospitalized in our center and since we need much more time for the follow-up, still cannot conclude clearly about the prognosis. Also a very important question occurs to us: is there any relationship between ARVD and LVNC, at least, at their genetic loci? The answer to this question may clarify why some types of the former, in the context of the presence of skin lesions, manifest chiefly in the left ventricle while in the other types, it is the right ventricle which plays the role of main victim. This case does confirm that dilated cardiomyopathy’s spectrum is wider than ever known and that like what happened at the congress of Boston in 2006 (15), a more comprehensive approach to its genetic types needs to be done.