1. Background
Diabetes mellitus is one of the most common endocrine disorders all over the world. It is estimated that 1 in 11 adults suffer from diabetes and gestational diabetes mellitus an impact of 1 in every 7 births (1). Abortion and congenital malformations occur as a result of poor glycemic control during contraception and first trimester (2, 3). Cardiac muscle hyperplasia, disorganized pattern of myofibrosis (4) as well as activation of chronic inflammatory pathways by placental genes (5) are caused by maternal diabetes which brings about cardiac performance alteration and increased vulnerability to stress (6). Cardiac muscle contractility abnormalities, hyperbilirubinemia, neonatal hypoglycemia (7, 8) and diastolic dysfunction caused by impaired ventricular compliance (9) are the reported complications for the fetuses of the diabetic mothers. Fetal echocardiography plays a role as a non-invasive modality for the evaluation of fetal heart anatomy, function and hemodynamics (10). Diastolic function can be assessed by Doppler echocardiography (11) and ventricular myocardial contraction and expansion during systole and diastole by tissue Doppler echocardiography (12, 13). Not affected by ventricular geometry and heart rate and including both systolic and diastolic parameters, myocardial performance index (MPI) has been emerged as useful Doppler index for the global cardiac function evaluation (10). E/A velocities at mitral and tricuspid valves are the tools for diastolic function assessment (14). Pre load index, first described by Kanzaki and Chiba (15), is an index which represents the right atrial pressure, by using the flow velocities in Inferior Vena Cava, reflecting the preload status of the heart.
2. Objectives
This study was conducted to investigate fetal cardiac ventricular function by evaluating preload index, MPI, E/A ratio, ventricular septal thickness and cardiac output and to determine which is more beneficial for detecting early myocardial changes.
3. Methods
3.1. Study Population
The study was conducted as an observational cross-sectional study, at a tertiary referral center, Children's Medical Center, Tehran, Iran. Between March 2016 and March 2017, 25 consecutive women with gestational diabetes mellitus and 50 healthy pregnant women were recruited. The evaluations were performed in 18 - 23 weeks of gestation. The diagnosis of the gestational diabetes was based on WHO criteria, with fasting plasma glucose < 7.0 mmol/L, 2-hour post prandial glucose level ± 7.8 mmol/L and < 11.1 mmol/L after ingestion of 75 gram glucose for tolerance test (16). The exclusion criteria were multiple pregnancies, congenital malformations, abnormal fetal heart rate, maternal systemic hypertension, systemic lupus erythematous, pregnancy-induced hypertension, pre-eclampsia, eclampsia, chromosal abnormalities, intrauterine growth restriction and pregnancy by assisted reproductive technology and any drugs prescribed for mother other than routine vitamins. The control group was selected among healthy patients with no remarkable obstetric history. HbA1c was controlled in all the participants, the level of which was below 6 in both cases and controls. The drug history was negative for Aspirin. The study was approved by our institutional review board and informed consent was obtained from all participants.
3.2. Imaging Protocol
All imaging studies were performed by a single physician using Philips affinity 70 w Ultrasound machine, with the convex probe (2 - 9 Hz). The structural abnormality was ruled out by an initial systemic 2-dimensional fetal echocardiography with high frame rate (60 - 150 frame/sec), the existence of malformations was checked in all standard views. The segmental sequential approach was taken for a complete fetal echocardiographic examinations (17). Three or more waveforms were recorded using pulsed Wave Doppler (PW), with isonation angle < 20 degrees.
3.3. Myocardial Performance Index
The MPI index was calculated for both right and left heart by PW Doppler. Three intervals were recorded for each index: Isovolumetric contraction time (ICT), isovolumetric relaxation time (IRT) and ejection time (ET). ICT is the period between mitral/tricuspid valve closure and aortic/pulmonary valve opening. IRT is the period between aortic/pulmonary valve closure and mitral/tricuspid valve opening and ET is the period between aortic/pulmonary valve opening and closure. Mod-MPI = (ICT + IRT)/ET.
3.4. Pre-Load Index
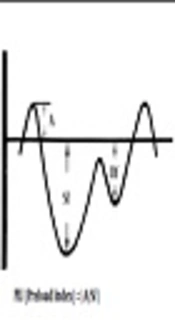
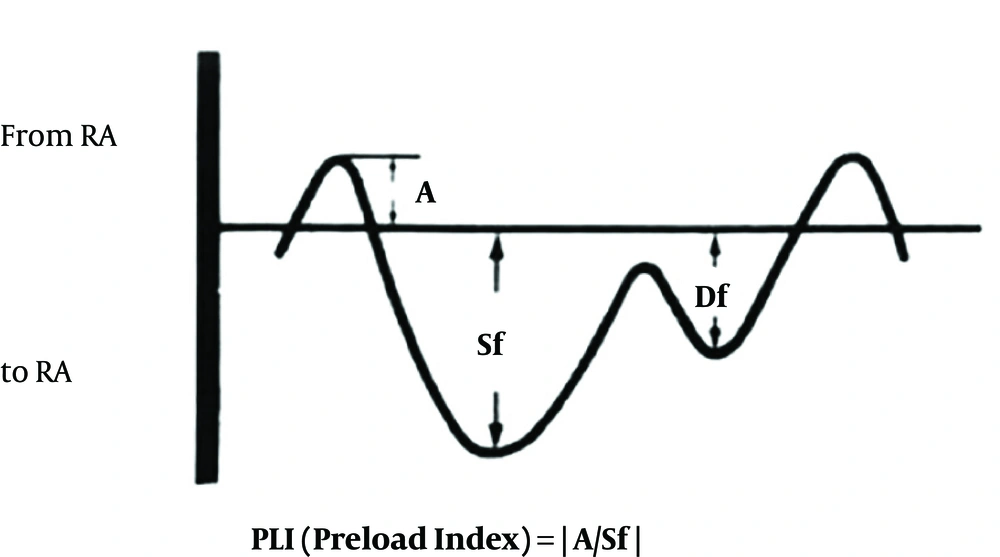
This index was recorded during the periods of fetal apnea, when there were no breathing movements. With the observation of blood flow in IVC, the sampling volume was placed as close as possible to the heart. The best alignment of the Doppler for PLI determination could be obtained from longitudinal view of the IVC, just below the RA-IVC junction. Care must be taken not to be mistaken by the hepatic vein flows. Ductus venous flow may sometimes interfere with this investigation. Doppler flow optimization is necessary to get the best possible results. Doppler gain should be adjusted, so there will be no unnecessary noises. The Doppler envelope should fill two thirds of the imaging plane. The reverse flow (A wave) during atrial contraction, the initial forward flow (Sf wave) during atrial relaxation and ventricular contraction, second forward flow (Df wave) during ventricular diastole were observed. The preload index was defined as A/Sf (Figure 1) (15).
3.5. Inter-Ventricular Septal Thickening
Using the M-mode echocardiography during the diastole, the Inter-ventricular septal thickening was measured by placing the M-line perpendicular to Inter-ventricular septum, just below the aortic valves in transverse five-chamber view during the suspended voluntary maternal respiration with no fetal breathing movements.
3.6. Cardiac Output
Right and left side cardiac output was calculated by using the following formula:
Left side cardiac output = 0.785 × (aortic valve diameter)2 × VTI × HR
Right side cardiac output = 0.785 × (pulmonic valve diameter)2 × VTI × HR
Where VTI means velocity time integral and HR means heart rate.
3.7. Statistical Analysis
The data was analyzed with SPSS software version 22. The quantative values were expressed with mean ± SD and P value less than 0.05 was considered significant. The normality of the parameters distribution pattern was evaluated by Kolmogorov Smirnov test. The t-test was used for normally distributed ones; otherwise the Mann-Whitney test was used.
4. Results
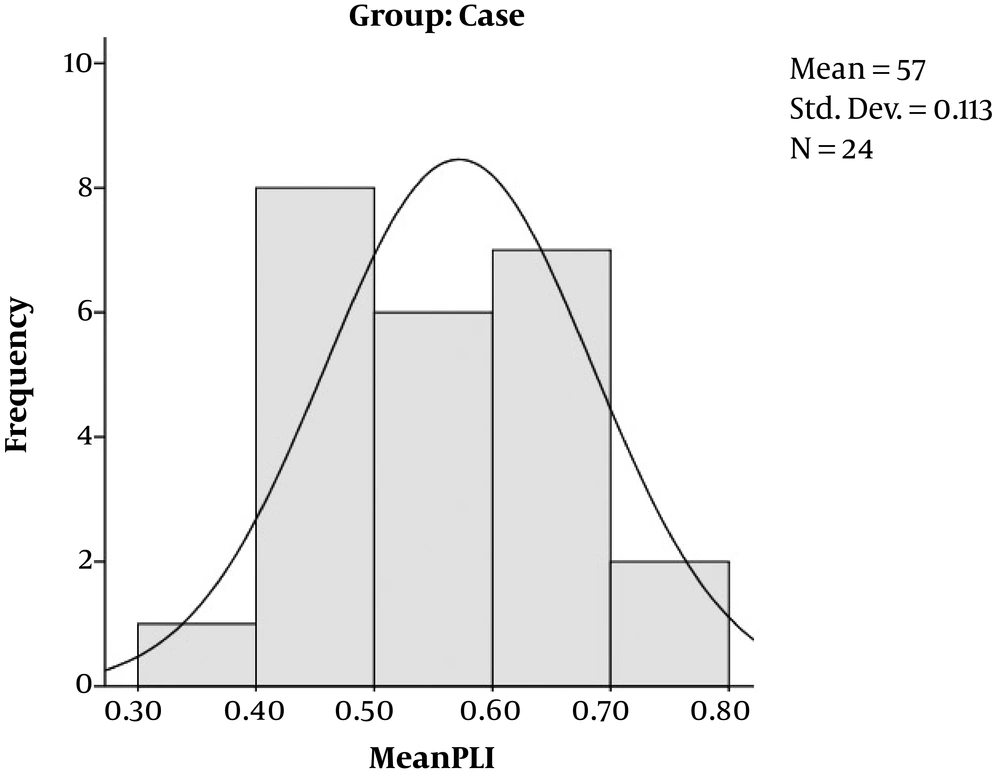
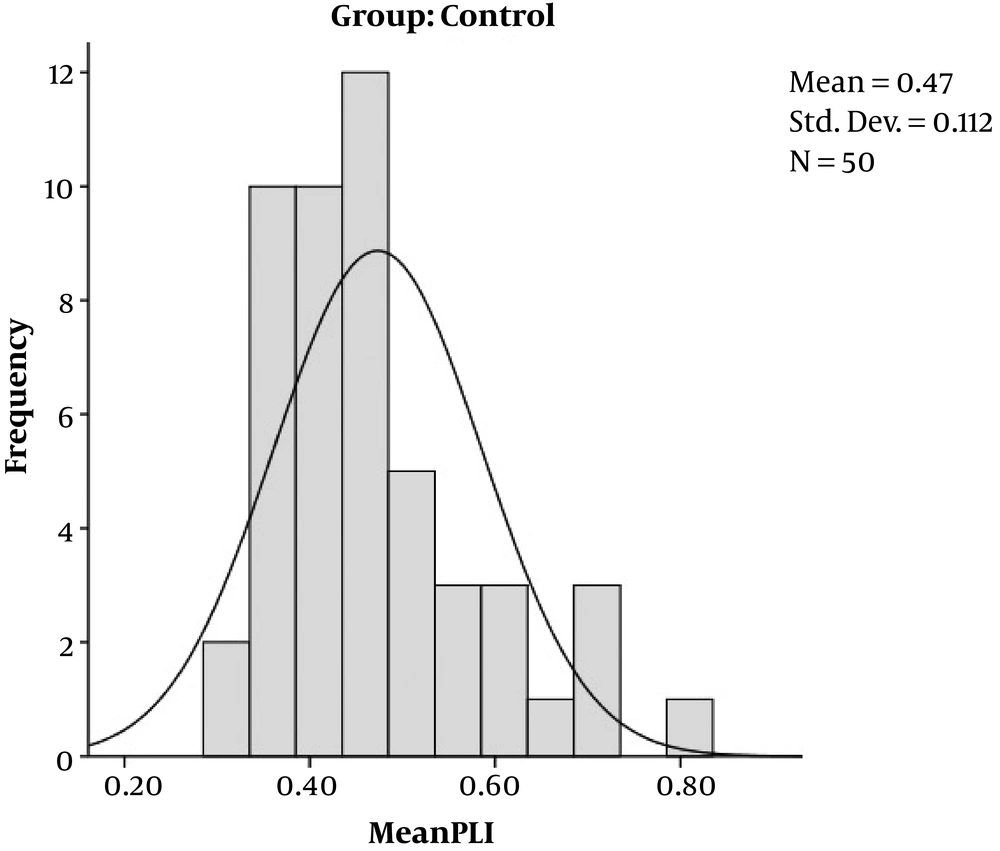
This study was conducted on 25 cases, pregnant women with gestational, and on 50 healthy pregnant women without any history of diabetes, who came to our institution for routine check-up. All the case group had good glycemic control. Male gender of the fetuses had a greater portion in control group (67.40% vs. 65.60%) but female gender in case group (34.40% vs. 32.60%). The mean gestational age was 33 ± 5 weeks, the case group had 31 ± 8 weeks of gestation and control 31 ± 5 weeks, without any significant difference (P = 0.994). The left ventricular MPI and Right ventricular MPI did not differ statistically between the cases and controls (P = 0.359 and 0.961). Cases had a greater interventricular wall thickness in comparison to controls (P = 0.044). The right sided and left sided cardiac output did not differ significantly between the cases and controls (P = 0.624 and 0.329). The PLI value was greater in the case group (0.57 ± 0.11) compared to the control group (0.47 ± 0.11) with P value of 0.001 (Table 1, Figures 2 and 3).
| Case Group | Control Group | P Value | |
|---|---|---|---|
| Left MPI | 0.43 ± 0.09 | 0.48 ± 0.27 | 0.359 |
| Right MPI | 0.45 ± 0.11 | 0.46 ± 0.32 | 0.961 |
| Interventricular septal thickness | 0.23 ± 0.07 | 0.20 ± 0.04 | 0.044 |
| Left cardiac output | 741.76 ± 391.98 | 612.60 ± 517.90 | 0.329 |
| Right cardiac output | 904.81 ± 731.72 | 821.92 ± 710.4 | 0.624 |
| PLI | 0.57 ± 0.11 | 0.47 ± 0.11 | 0.001 |
Abbreviations: MPI, myocardial performance index; PLI, pre-load index.
a Values are expressed as mean ± SD.
5. Discussion
Cardiac function impairment in the fetuses of the diabetic mothers during the second and third trimester is well established (14). Organogenesis problems with defects in neural crest migration during the first trimester will lead to structural conotruncal defects (18); however, in the second and third trimester myocardial hypertrophy and myocardial dysfunction occur as a result of hyperglycemia, with a 5-time greater risk of still birth (19), moreover, the alteration in myocardial contractile protein isoforms befalls in the second trimester (20). Fetal echocardiography has emerged as a great progress for prenatal diagnosis of congenital heart disease that has made the intra-uterine treatment possible as well. Cardiac function changes in the sonographic parameters emerge even sooner than cardiac structure ultrasonic changes (21). Diastolic dysfunction is considered as the earliest change in fetal myocardium which proceeds to systolic dysfunction and finally symptomatic heart failure (21).
In 1996, a new modality named myocardial performance index has been introduced for the assessment of heart failure (22). It is a useful tool in the pediatric population for the cardiac evaluation of the patients undergoing anthracycline therapy (23), single ventricle physiology (24) and candidates for heart transplant (25). It has been shown that the MPI is increased in the fetuses of the diabetic mothers, independent of onset of maternal diabetes but dependent of the insulin usage (26); although the results of our study showed no difference in both right and left side MPI. According to Tsutsumi et al. (27), the fetuses of diabetics have higher level of MPI in 27 to 40 weeks of gestation, compared with controls. Similar result has been reported by Balli et al. (28), demonstrating greater left ventricular MPI in the fetuses of the women with gestational diabetes; however, Ichizuka et al. (29) have shown that this index is increased in large-for-gestational fetuses of diabetics, and not in the appropriate-for-gestation or small-for-gestation fetuses. On the other hand, according to a recent study by Pilania et al. (30), MPI at 26 - 28 weeks and cardiac output at 34 - 36 weeks of gestation can predict the adverse outcomes. Despite all the previous reports, our study did not approve any abnormalities in MPI of the fetuses of diabetic mothers. Previous studies have propounded fetal progressive cardiac output decrement through the pregnancy of diabetics (31). In our patients, the cardiac output, both in the right and left side, were similar to the healthy ones.
Having a pulsatile pattern, blood flow of inferior vena cava (IVC) is synchronized with cardiac cycle (32). IVC blood flow patterns can be detected by pulsed Doppler echocardiography in the fetuse (33). Using IVC pulsed Doppler echocardiography, the PLI index can be calculated, representing preload state of the fetus heart (15). PLI value increment is associated with several conditions such as tricuspid regurgitation or some structural heart diseases (15), lower umbilical atrial blood pH, higher hematocrit at the time of the birth and increased risk of neonatal morbidity (34). Up to now, several indices, such as MPI and E/A ratio, have been suggested for early detection of diastolic dysfunction, as the primary sign of proceeding to heart failure. As far as we know, diastolic heart failure leads to increased cardiac preload, thus Increased PLI index, which reflects diastolic dysfunctions. In our study, in contrast to the previous studies, the MPI did not differ between the fetuses of the gestational diabetic mothers and non-diabetic ones; but, fetuses of gestational diabetic mothers had a greater value of PLI, representing early changing in diastolic function in the right heart even before the heart failure occurs. This could be a sign of vasculopathy in gestational diabetic mothers.