1. Background
Memory impairment diseases encompass conditions that hinder a person’s ability to retain information. Some common examples of memory impairment diseases include Alzheimer’s disease (AD), dementia, Parkinson’s disease, Huntington’s disease, and traumatic brain injury (1). Alzheimer’s disease is a chronic neurodegenerative condition that typically manifests slowly and progressively worsens. It is an incurable, debilitating, and often fatal disease that primarily affects individuals over the age of 65 years. Alzheimer’s disease accounts for 60% to 70% of dementia cases, with the most common initial symptom being short-term memory impairment and difficulty in recalling new information and recent events (2). In AD, there is a disruption in the transmission of messages in the cholinergic nerves of the brain. Furthermore, a decrease in the number of cholinergic neurons and a reduction in acetylcholine transferase activity in the cerebral cortex and hippocampus of Alzheimer’s patients have been reported (3).
Scopolamine (SCP) is a muscarinic receptor antagonist that induces temporary memory impairment. Studies have demonstrated numerous similarities between memory deficits in Alzheimer’s patients and animals treated with scopolamine, making scopolamine a valuable tool for creating an animal model of AD in pharmacological research (4).
Recent studies have shed light on the role of diabetes in the development of AD. As individuals become resistant to insulin, their brain tissue’s glucose tolerance diminishes, hindering optimal glucose utilization by the brain. This disorder can lead to nerve cell death and a decline in the brain’s ability to process messages (5). Moreover, beta-amyloid (Aβ) plaques, which accumulate in the brains of Alzheimer’s patients, interfere with the normal functioning of insulin receptors, reducing the brain tissue’s sensitivity to insulin. Type 2 diabetes is now considered a significant risk factor for AD, with the prevalence of AD in type 2 diabetes patients reported to range from 60% to 70% (6).
Additionally, oxidative stress plays a pivotal role in the pathogenesis of AD. Oxidative stress arises when there is an imbalance between the production of reactive oxygen species (ROS) and their detoxification, resulting in damage to cellular components, such as proteins, lipids, and deoxyribonucleic acid (DNA). The accumulation of Aβ protein in the brain is believed to trigger oxidative stress, leading to neuronal damage and inflammation. Furthermore, oxidative stress might contribute to the formation of fibrillary tangles, a hallmark of AD (7, 8).
Sitagliptin (SG) and metformin (MTF) are anti-hyperglycemic medications utilized to regulate blood glucose levels in diabetic patients. These drugs operate by enhancing insulin secretion, augmenting the sensitivity of pancreatic alpha and beta cells to insulin, increasing beta cell mass and insulin production, reducing the rate of gastric emptying and appetite, enhancing insulin receptor sensitivity, and decreasing glucagon secretion from the pancreas (9, 10). Previous studies have demonstrated that SG and MTF can influence the symptoms of AD by regulating blood glucose levels and promoting the enhancement and fortification of insulin function (11, 12).
2. Objectives
This study was designed to investigate the effect of SG and MTF on learning and memory impairment induced by scopolamine in diabetic and non-diabetic mice.
3. Methods
3.1. Drugs and Chemicals
Sitagliptin, MTF, SCP, and streptozotocin (STZ) with a purity greater than 99% were purchased from Sigma-Aldrich Co (St. Louis, MO, USA). All other chemicals used were of analytical grade.
3.2. Animals
BALB/c male albino mice weighing 25 - 30 g were utilized for the study. The animals were obtained from the Animal House of Mazandaran University of Medical Sciences, Sari, Iran, and were maintained at a temperature of 23 ± 2°C with a 12-hour light/dark cycle. Food and water were consistently provided to the animals, except during the experiments, and each animal was used only once.
3.3. Experimental Protocol
The present study was conducted in two sections involving diabetic and non-diabetic mice. In the non-diabetic section, the mice were randomly divided into 8 groups, with each group consisting of 8 animals. These groups were as follows:
Group 1 (control): The animals received normal saline for 14 successive days.
Group 2 (positive control): The animals received SCP (20 mg) on the 14th day.
Groups 3 and 4: The animals received SG at doses of 10 mg and 20 mg, respectively, and SCP (20 mg) on the 14th day.
Groups 5 and 6: The animals received MTF at doses of 250 mg and 500 mg, respectively, and SCP (20 mg) on the 14th day.
Group 7: The animals were given SG (10 mg/kg) and MTF (250 mg/kg) for 14 successive days, along with 20 mg/kg of SCP on the 14th day.
Group 8: The animals were given SG (20 mg/kg) and MTF (500 mg/kg) for 14 successive days, along with 20 mg/kg of SCP on the 14th day.
In the diabetic section, blood sugar was measured before the injection of STZ (time 0) and 2 weeks after STZ treatment using an Accu-Chek Active glucometer (Roche, Switzerland). The mice with blood sugar levels above 150 mg/dL were selected for testing and randomly divided into 5 groups, each consisting of 8 animals. These groups were as follows:
Group 1 (control): Diabetic mice received normal saline for 14 consecutive days.
Group 2 (positive control): Diabetic mice were treated with SCP (20 mg) on the 14th day.
Group 3: Diabetic mice received MTF (500 mg/kg) for 14 successive days, along with 20 mg/kg of SCP on the 14th day.
Group 4: Diabetic mice received SG (20 mg/kg) for 14 successive days, along with 20 mg/kg of SCP on the 14th day.
Group 5: Diabetic mice received SG (20 mg/kg) and MTF (500 mg/kg) for 14 successive days, along with 20 mg/kg of SCP on the 14th day.
All drug doses were administered intraperitoneally daily for 14 consecutive days. On the 14th day, the memory recall test was conducted 45 minutes after the last administration using a shuttle box apparatus (13).
3.4. Diabetes Induction
To induce diabetes mellitus in mice, a dose of 90 mg/kg of STZ was administered intraperitoneally for 2 consecutive days following an overnight fasting period (without food). One week after the STZ treatment, blood samples (0.1 mL) were collected from the mice, and their glucose levels were measured using a glucometer that was approved by the United States Food and Drug Administration (FDA) and the reference laboratory in Iran (Accu-Chek Active, Roche, Switzerland). The mice with glucose concentrations higher than 180 mg/dL after receiving STZ were considered diabetic mice (13, 14).
3.5. Behavioral Test
3.5.1. Memory Assessments and Passive Avoidance Learning
The experiment was conducted in three stages using a shuttle box apparatus.
The shuttle box apparatus consists of two plexiglass chambers, one light and one dark, each with dimensions of 20 × 20 × 40 cm. These chambers are connected by an 8 × 8 cm opening. Both sections have stainless steel metal bars on the floor spaced 1 cm apart, through which an electric shock can be delivered to the animal’s feet when in the dark chamber. A 60-watt lamp was used to illuminate the light chamber.
Stage 1. Acclimatization: Initially, all the animals were familiarized with the shuttle box apparatus. Mice from each group were individually placed in the shuttle box, and after 5 seconds, the door separating the two chambers was opened, allowing the mice to explore both the dark and light chambers freely for 2 minutes. Typically, mice tend to naturally move into the dark chamber during this stage. As soon as a mouse entered the dark area, the door was closed, and the mice were returned to their cages.
Stage 2. Acquisition: Thirty minutes after the acclimatization phase, the acquisition phase began. Initially, the mice were placed in the well-lit section of the box, and they were given 2 minutes to enter the dark compartment. Once a mouse entered the dark area, the door was closed, and an electric shock with a frequency of 50 Hz and an intensity of 1.2 mA was applied to the animal’s leg for 1.5 seconds. The mouse was then removed from the dark chamber and returned to its cage. After a 2-minute interval, the mouse was placed back in the well-lit area to assess learning. Successful learning was defined as not re-entering the dark area within 120 seconds. If the mouse re-entered the dark compartment, the door was closed a second time, and the shock was applied as before. This process was repeated until the mice learned not to enter the dark compartment. The number of attempts required for each mouse to learn was recorded.
Stage 3. Retention: The retention test was conducted 24 hours after the training. During this phase, the mouse was placed in the well-lit compartment, and 5 seconds later, the door to the dark compartment was opened. The time it took for the animal to enter the dark compartment (step-through latency) and the duration it remained there (time spent in the dark compartment) were recorded for a period of 10 minutes (15, 16).
3.6. Sample Collection
Immediately after the behavioral test, the animals were anesthetized using a combination of ketamine (100 mg/kg, intraperitoneal) and xylazine (10 mg/kg, intraperitoneal). Subsequently, the animals’ skulls were opened, and their brains were rapidly isolated. The brain tissue was preserved at -70°C and later utilized for tissue antioxidant assays.
3.7. Biochemical Assay
For this purpose, brain tissue was initially homogenized in a 0.1 M phosphate buffer with a pH of 7.4 at a concentration of 10% using an FSH-2A homogenizer. Subsequently, it was centrifuged at 1000 rpm for 10 minutes using a refrigerated centrifuge (Hettich Zentrifugen, Germany) to remove unbroken cells and cell debris. The Bradford method was employed to assess the protein content of tissue homogenates (17). The tissue homogenate was then utilized to measure oxidative stress factors, including malondialdehyde (MDA), glutathione (GSH), glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD).
3.8. MDA and GSH Measurement
In the present study, the Buege and Aust method (18) was employed to determine the MDA levels. Malondialdehyde concentration was determined using tetraethoxypropane as a standard, and the results were expressed in nmol/mg of protein. Glutathione content was measured using the Ellman method (19). A GSH standard was used to create the calibration curve, and GSH content was expressed in nmol/mg of protein.
3.9. SOD, CAT, and GPx Activity Assay
ZellBio commercial kit was used to measure CAT, SOD, and GPx activity. These kits use standard protocols for measuring the aforementioned enzymes (20, 21). Additionally, the activities of CAT, SOD, and GPx were expressed as units per gram of tissue.
3.10. Ethics Statement
This experimental study was conducted according to the Animal Ethics Committee Guidelines of Mazandaran University of Medical Sciences, Sari, Iran (ethics code: IR.MAZUMS.REC.1399.633).
3.11. Statistical Analysis
Statistical analysis was conducted using GraphPad Prism software (version 6). The results were presented as mean ± standard error of the mean (SEM). The normality of data distribution was assessed using the Kolmogorov-Smirnov test. One-way analysis of variance (ANOVA) was employed to compare the means of the groups, and post hoc analysis was performed using Tukey’s test. A difference with a P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Effect of Metformin and Sitagliptin on Learning and Memory Retrieval
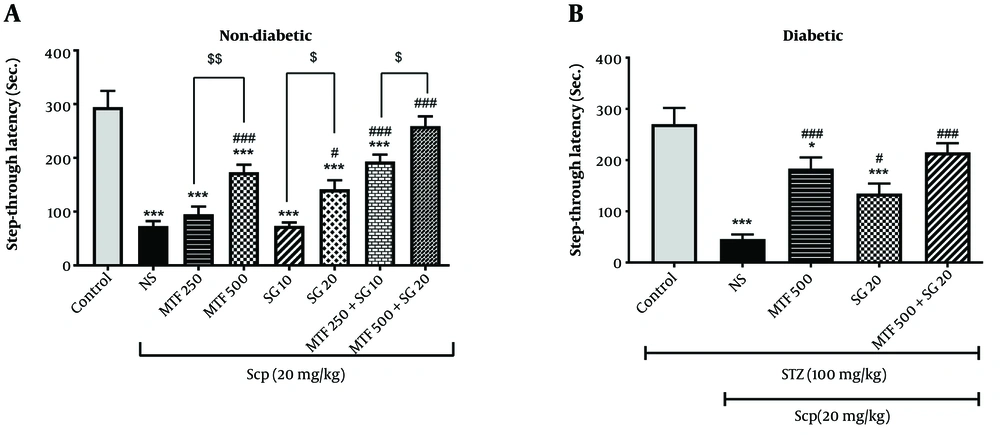
The current study’s findings indicated that the administration of SCP in both diabetic and non-diabetic animals significantly decreased step-through latency compared to the control group (P < 0.001). In non-diabetic mice, the administration of high doses of MTF and SG (500 and 20 mg/kg, respectively) significantly increased this parameter compared to the SCP group (P < 0.001). Additionally, treatment with the combination of MTF and SG in groups 7 and 8 significantly reversed the memory impairment induced by SCP (P < 0.001). Notably, group 8, which was treated with a high dose of MTF and SG (500 mg/kg MTF + 20 mg/kg SG), showed the most favorable results (Figure 1).
Effect of metformin (MTF) and sitagliptin (SG) on learning and memory retrieval in passive avoidance test. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, *** P < 0.001). # Significantly different from the scopolamine (SCP) group (# P < 0.05, ### P < 0.001). $ Significant difference between treatment groups ($ P < 0.05, $$ P < 0.01).
In diabetic mice, the administration of MTF (500 mg/kg), SG (20 mg/kg), and the combination of MTF and SG (500 and 20 mg/kg, respectively) significantly increased step-through latency compared to the SCP group. Moreover, the best results were observed in the groups receiving MTF and the combination of MTF and SG (P < 0.001) (Figure 1).
A comparison between the similar treatment groups in diabetic and non-diabetic mice showed a slight elevation of this parameter in diabetic animals compared to non-diabetic animals. However, this increase was not statistically significant (P > 0.05).
4.2. Effect of Metformin and Sitagliptin on Tissue Oxidative Stress Parameters
In the present study, to gain a deeper understanding of the mechanism by which MTF and SG improve learning and memory impairment, this study evaluated oxidative stress parameters in brain tissue. Specifically, we measured MDA levels, GSH content, and SOD, GPx, and CAT activities in brain tissue (Figures 2-5).
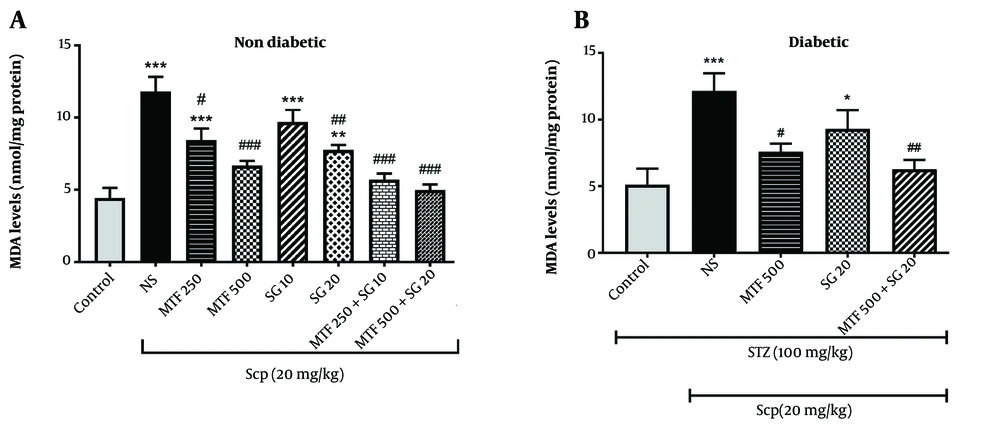
Effect of metformin (MTF) and sitagliptin (SG) on malondialdehyde (MDA) level each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P < 0.001). # Significantly different from the scopolamine (SCP) group (# P < 0.05, ## P < 0.01, ### P < 0.001).
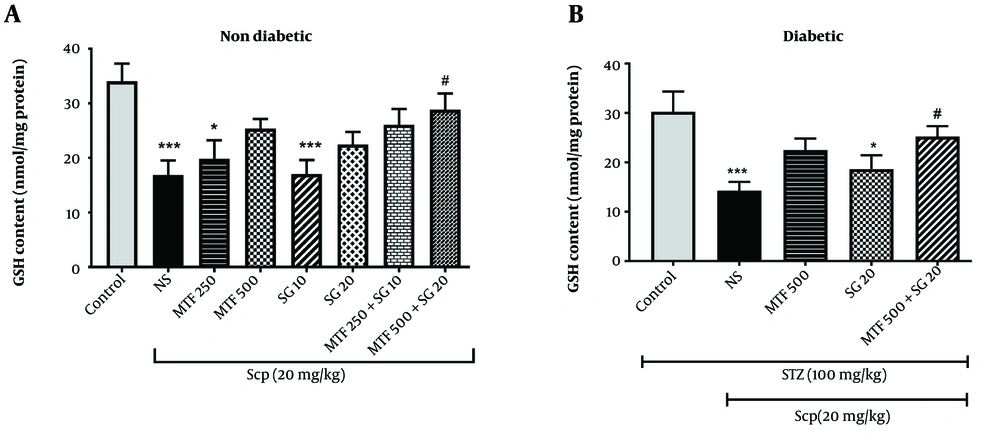
Effect of metformin (MTF) and sitagliptin (SG) on glutathione (GSH) content each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, *** P < 0.001). # Significantly different from the scopolamine (SCP) group (# P < 0.05).
Pre-treatment effects of metformin (MTF) and sitagliptin (SG) on antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], and glutathione peroxidase [GPx]) in non-diabetic mice. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P< 0.001). # Significantly different from scopolamine (SCP)-treated group (# P < 0.05, ## P < 0.01, ### P < 0.001).
Pre-treatment effects of metformin (MTF) and sitagliptin (SG) on antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], and glutathione peroxidase [GPx]) in diabetic mice. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P < 0.001). # Significantly different from the scopolamine (SCP)-treated group (# P < 0.05, ### P < 0.001).
4.2.1. Effect of Metformin and Sitagliptin on Malondialdehyde Level in Brain Tissue of Mice Exposed to Scopolamine
In terms of lipid peroxidation, the current study’s findings revealed that SCP administration in both diabetic and non-diabetic animals significantly increased MDA levels compared to the control group (P < 0.001). In non-diabetic mice, the administration of high doses of MTF and SG (500 and 20 mg/kg, respectively) significantly decreased MDA levels compared to the SCP group (P < 0.001 and P < 0.01, respectively) (Figure 2). Treatment with the combination of MTF and SG in groups 7 and 8 also significantly decreased MDA levels compared to the SCP group (P < 0.001). Moreover, there was no significant difference between groups 7 and 8 (P > 0.05).
In diabetic mice, the administration of MTF (500 mg/kg) and the combination of MTF and SG (500 and 20 mg/kg, respectively) significantly decreased MDA levels, compared to the SCP group (P < 0.05 and P < 0.01, respectively) (Figure 2). Comparing similar treatment groups in diabetic and non-diabetic mice revealed that this parameter was slightly higher in diabetic animals than in non-diabetic animals; however, this increase was not significant (P > 0.05).
4.2.2. Effect of Metformin and Sitagliptin on Glutathione Content in Brain Tissue of Mice Exposed to SCP
The results indicated that the administration of SCP in both diabetic and non-diabetic animals significantly reduced GSH content compared to the control group (P < 0.001) (Figure 3). In non-diabetic mice, the administration of high doses of MTF and SG (500 and 20 mg/kg, respectively) and a combination of MTF and SG (250 and 10 mg/kg, respectively) led to a slight increase in GSH content compared to the SCP group (P > 0.05). However, treatment with the combination of high doses of MTF and SG (500 and 20 mg/kg, respectively) in group 8 significantly increased GSH content compared to the SCP group (P < 0.001) (Figure 3). In diabetic mice, pretreatment with a combination of MTF and SG (500 and 20 mg/kg, respectively) significantly increased GSH content compared to the SCP group (P < 0.05) (Figure 3).
4.2.3. Effect of Metformin and Sitagliptin on Antioxidant Enzymes Activity in Brain Tissue
Comparison of antioxidant enzyme activity in different groups revealed that SCP administration in both diabetic and non-diabetic animals significantly decreased the activities of SOD, GPx, and CAT compared to the control group (P < 0.001) (Figures 4 and 5). When measuring the activity of antioxidant enzymes in non-diabetic mice, it was observed that the administration of MTF (250 and 500 mg/kg), SG (20 mg/kg), and a combination of MTF and SG (250 and 10 mg/kg, 500 and 20 mg/kg) significantly increased GPx activity, compared to the SCP group (P < 0.001). Additionally, pretreatment with MTF (500 mg/kg) and the combination of MTF and SG (250 and 10 mg/kg, 500 and 20 mg/kg) significantly elevated CAT activity in brain tissue, compared to the SCP group (P < 0.001). Moreover, pretreatment with MTF (500 mg/kg), SG (20 mg/kg), and the combination of MTF and SG (250 and 10 mg/kg, 500 and 20 mg/kg) significantly increased SOD activity in comparison to the SCP group (P < 0.001) (Figure 4).
In diabetic mice, the group receiving a combination of MTF and SG (500 and 20 mg/kg, respectively) showed a significant increase in GPx activity compared to the SCP group (P < 0.05). Regarding CAT and SOD activities, pretreatment with MTF (500 mg/kg) and the combination of MTF and SG (500 and 20 mg/kg) significantly elevated the activities of these enzymes in brain tissue, compared to the SCP group (P > 0.05 and P < 0.001, respectively) (Figure 5). Furthermore, when comparing similar treatment groups in diabetic and non-diabetic mice, it was observed that the levels of GSH, CAT, GPX, and SOD in diabetic animals were lower than in non-diabetic animals, although this difference was not statistically significant (P > 0.05).
5. Discussion
In the current study, we assessed the impact of SG and MTF as single and combined treatments on scopolamine-induced learning and memory impairment in both diabetic and non-diabetic mice. The results revealed that the intraperitoneal injection of 20 mg/kg of SCP induced learning and memory impairment in both healthy and diabetic mice. These findings align with prior research, where the administration of SCP was observed to increase oxidative stress and induce memory impairment in mice (22, 23).
There is compelling evidence suggesting that certain antidiabetic drugs, such as MTF and SG, might have a beneficial effect in the treatment of AD. These drugs have been shown to reduce brain inflammation and improve cognitive function in animal studies (24-26). Although there is limited research on the combined use of MTF and SG specifically for improving cognitive function, both drugs have individually demonstrated potential benefits for cognitive function and memory impairment. Metformin has been shown to enhance cognitive function in some studies, possibly by reducing brain inflammation and oxidative stress (27). Sitagliptin has also been shown to mitigate neuroinflammation and oxidative stress in the brain while enhancing neurotransmitter levels (28, 29).
The present study’s findings indicated that in healthy mice, high doses of MTF (500 mg/kg) and SG (20 mg/kg) and their combination (MTF + SG) significantly improved learning and memory impairment induced by SCP. In the second stage of the research involving diabetic mice, intraperitoneal injections of MTF and SG, both individually and in combination, improved the learning and memory impairment in diabetic mice. However, the combination of MTF (500 mg/kg) with SG appeared to have a more pronounced effect.
Oxidative stress is another factor that plays a crucial role in the development and progression of memory impairment. In the brain, oxidative stress can impair neuronal function and communication, disrupt synaptic plasticity, and promote neuroinflammation. These effects can ultimately lead to cognitive deficits and memory impairment. Therefore, reducing oxidative stress might be a potential strategy for preventing or treating memory impairment (30, 31).
In the present study, various biomarkers of oxidative stress, including MDA, GSH, CAT, SOD, and GPX, were measured. Given that SCP toxicity generates reactive oxygen metabolites, the measurement of MDA and GSH content can be valuable in diagnosing SCP neurotoxicity. The results demonstrated that SCP administration significantly increased MDA levels and depleted GSH content in brain tissue, indicating oxidative stress. These findings are consistent with other studies’ findings that have reported significant GSH depletion and MDA elevation due to SCP intoxication (32, 33).
In this study, pretreatment with different doses of MTF and SG restored GSH content, with the most significant effect observed in the combination of SG (20 mg/kg) and MTF (500 mg/kg). Regarding MDA, high doses of SG (20 mg/kg), MTF (500 mg/kg), and the combination of these drugs significantly reduced MDA formation in healthy mice. However, in diabetic mice, only the administration of 500 mg/kg MTF and the combination of MTF and SG significantly reduced MDA formation.
The antioxidant system of living organisms consists of non-enzymatic antioxidants, such as GSH, and endogenous antioxidant enzymes, such as GPx, SOD, and CAT, which protect cells against oxidative stress (34). The current study’s results revealed a significant decrease in the activities of antioxidant enzymes, GPx, CAT, and SOD, in the brain tissues of SCP-treated mice, compared to the control group, indicating that SCP induced severe oxidative stress.
Furthermore, the administration of scopolamine in diabetic mice resulted in slightly more oxidative stress and memory impairment than in healthy mice. This finding highlights the potential role of diabetes as a risk factor in the development of neurological disorders. In non-diabetic mice, pretreatment with high doses of MTF and SG, in addition to combinations of SG and MTF, significantly increased GPx, CAT, and SOD activity. However, in diabetic mice, the administration of only 500 mg/kg MTF and the combination of MTF and SG restored antioxidant enzyme activity.
Overall, the results of this study demonstrated that in healthy mice, both high doses of MTF and SG, in addition to the combination of these two drugs, significantly reduced oxidative stress. However, in the diabetic group, only MTF and the combination of MTF with SG were able to reduce oxidative stress. Therefore, the authors conclude that these drugs might reduce oxidative stress and inhibit lipid peroxidation in brain tissue by increasing its antioxidant capacity. These findings are consistent with previous studies’ findings. For example, in a study by Zhao et al., intraperitoneal injection of 200 mg/kg MTF for 14 days suppressed the development of chemical kindling created by pentylenetetrazol (PTZ), reduced brain oxidative stress and improved cognitive impairment caused by PTZ (35). These results are also in line with previous research conducted by Alzoubi et al., who demonstrated that oral gavage of MTF over a period of 4 weeks in rats prevented cognitive damage caused by L-methionine by reducing oxidative stress in the hippocampus (36).
In another study by Civantos et al., the role of SG in oxidative stress and its underlying mechanisms were investigated in diabetic rats. The aforementioned study’s findings indicated that SG effectively reduced oxidative stress in experimental diabetic nephropathy through the downregulation of miR-200a, a novel Keap-1 inhibitor, and miR-21 (37).
Several mechanisms have been mentioned in relation to the beneficial effects of these drugs in improving memory impairment. Hettich et al. showed that metformin significantly reduces the activity and expression of the beta-secretase-1 enzyme in the cell culture medium, thereby reducing the products of the beta-secretase-1 enzyme (Aβ) (38).
Some researchers have suggested that MTF, due to its anti-inflammatory and anti-oxidative properties and its ability to reduce interleukin 1, can increase the survival of brain neurons and improve cognitive function (39). Additionally, Li et al. demonstrated that MTF treatment reduces the production of hyperphosphorylated tau proteins, one of the pathological signs of AD, in the brains of diabetic rats (40).
Recent studies have also indicated the potential beneficial effects of SG on cognitive function and memory impairment. Although the precise mechanism by which SG improves cognitive function and memory impairment is not fully understood, it is believed to work by reducing inflammation in the brain and enhancing the levels of certain neurotransmitters.
Brain inflammation is a common feature of many neurodegenerative diseases, including AD and Parkinson’s disease. Sitagliptin has been shown to reduce brain inflammation by inhibiting the activity of dipeptidyl peptidase-4 (DPP-4), an enzyme involved in the inflammatory response. By reducing inflammation, SG might protect brain cells from damage and improve cognitive function (28).
Additionally, SG has been observed to increase the levels of certain neurotransmitters in the brain, including acetylcholine and dopamine. Acetylcholine plays a role in learning and memory; nevertheless, dopamine is involved in motivation and reward. By boosting these neurotransmitter levels, SG might enhance cognitive function and memory (29). Dong et al. have suggested that SG activates two signaling pathways, glucagon-like peptide-1 and brain-derived neurotrophic factor-tropomyosin receptor kinase B (BDNF-TrkB), which are involved in protecting neurons and improving cognitive function (12).
Furthermore, the findings of this study indicated that combination therapy with MTF and SG was more effective than single therapy. Combination therapy is often employed in the treatment of complex diseases, such as cancer, uncontrolled diabetes, or hypertension (41, 42). Metformin and SG are commonly used in the treatment of type 2 diabetes mellitus. Metformin reduces glucose production in the liver and enhances insulin sensitivity; however, SG increases insulin secretion and reduces glucagon production. When used together, these drugs can act synergistically to improve glycemic control by targeting multiple pathways involved in glucose metabolism (43). Several studies have demonstrated that improved glycemic control is associated with the amelioration of cognitive impairment in patients with diabetes (44). Combination therapy might also reduce the risk of side effects associated with high doses of a single drug (42, 43).
5.1. Conclusions
The current study’s findings revealed that intraperitoneal injection of SCP in diabetic and healthy mice impaired learning and memory function and caused brain oxidative damage. However, the mentioned damages were more pronounced in diabetic mice. In healthy mice, the administration of MTF and SG in high doses, in addition to the combination of these two drugs, significantly reduces memory impairment and oxidative stress. However, in the diabetic groups, only MTF and the combination of MTF with SG could reduce memory impairment and oxidative stress. Finally, the authors concluded that these antidiabetic drugs reduced oxidative stress by increasing antioxidant capacity and improved scopolamine-induced memory impairment. Additionally, the combination of these two drugs was more fruitful.
![Pre-treatment effects of metformin (MTF) and sitagliptin (SG) on antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], and glutathione peroxidase [GPx]) in non-diabetic mice. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P< 0.001). # Significantly different from scopolamine (SCP)-treated group (# P < 0.05, ## P < 0.01, ### P < 0.001). Pre-treatment effects of metformin (MTF) and sitagliptin (SG) on antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], and glutathione peroxidase [GPx]) in non-diabetic mice. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P< 0.001). # Significantly different from scopolamine (SCP)-treated group (# P < 0.05, ## P < 0.01, ### P < 0.001).](https://services.brieflands.com/cdn/serve/3170b/71a08b8e636e444f2be3bc5df7e2523d97219cd9/ijpbs-138984-i004-F4-preview.webp)
![Pre-treatment effects of metformin (MTF) and sitagliptin (SG) on antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], and glutathione peroxidase [GPx]) in diabetic mice. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P < 0.001). # Significantly different from the scopolamine (SCP)-treated group (# P < 0.05, ### P < 0.001). Pre-treatment effects of metformin (MTF) and sitagliptin (SG) on antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], and glutathione peroxidase [GPx]) in diabetic mice. Each value represents the mean ± standard error of the mean (SEM) for 8 mice. * Significantly different from the control group (* P < 0.05, ** P < 0.01, *** P < 0.001). # Significantly different from the scopolamine (SCP)-treated group (# P < 0.05, ### P < 0.001).](https://services.brieflands.com/cdn/serve/3170b/5e38ab01b98cfeab824e5db20f991c9435706d2a/ijpbs-138984-i005-F5-preview.webp)