1. Background
Bipolar I disorder has lifetime prevalence of about 1% (1, 2). A high percentage of the patients are experiencing large amounts of relapses, residual symptoms and functional and cognitive dysfunctions (3). Mood disorders can significantly impair the performance of patients in the fields of employment, social relationships, and their quality of life (4, 5). Suicide risk among the patients with BID is up to 20 - 30 times greater than that of the general population (6-8). Despite medical advances, one of the most significant obstacles to improve the prognosis of patients with severe mental disorders is the medication noncompliance (9). About 20% - 70% of the patients with bipolar disorder have little interest in taking medications (10). Therefore, medication effects in a significant proportion of patients depend on the proper use of medication by the patients. Moreover, their attitude toward treatment is one of the factors that influence treatment compliance (11). A patient’s attitude toward the effects of the medications predicts the medication discontinuation and the treatment outcome (12-16). For an easy evaluation and validation of such attitudes, several scales are developed (17, 18), among them the 30-item drug attitude inventory (DAI) is one of the first and most widely used ones; and its 10-item version is as good as the original one in schizophrenia (19).
DAI is used in different studies. For example, in a study conducted on patients with schizophrenia, there was a significant relationship between DAI-30 scores, symptoms reduction, and less proportion of medication discontinuation.
In another study, there was a significant relationship between the DAI-30 scores and treatment adherence in the adolescents treated with psychiatric medications (20). In a study in the 1st episode of schizophrenia, DAI-30 cut off point was 20 in order to determine predictive validity (21). In another study DAI-10 scores in patients admitted to psychiatric ward were associated with understanding the need for medication but not with the daily use or the number of medications (22). It was also found that in the patients taking antipsychotics, the high score of DAI-10 was associated with the reduction of PANNS scores (23); moreover, the compliance therapy can improve DAI-10 score (24). In a study on the Chinese version of this tool, there was a positive relationship between the three components of the response to DAI-10 and compliance except for the psychotic patients. In addition, there was appropriate reliability for this instrument (25). The DAI-10 questionnaire was used in the bipolar disorder patients’ follow-up (BDPF) study (26).
2. Objectives
It was necessary to investigate the validity and reliability of the present instrument to evaluate the attitudes of Iranian patients. Thus, the current study aimed at evaluating the validity and the reliability of DAI-10 in the patients with BID and finding its relationship with patients’ compliance in order to predict this compliance.
3. Materials and Methods
3.1. Patients
The current qualitative study was part of a thesis-including the assessment of internal consistency and validity-derived from BDPF performed on patients, aged 18 to 57 years, from 2011 to 2012 in Tehran, Iran. These patients were hospitalized in Iran psychiatric hospital after 2010 diagnosed with bipolar disorder by faculty members; all the participants including 27 cases entered BDPF project. The 2nd part of the project (assessment of test-retest reliability) was conducted on the patients with bipolar I disorder hospitalized in Iran psychiatric hospital in 2012. Then, 82 patients of BDPF project were randomly selected based on the inclusion criteria. The inclusion criteria for research were: age 18 - 65 years, familiarity with the Persian language, access to the subjects` personal phones, diagnosis of bipolar I disorder before taking part in the study.
The exclusion criteria were: patients unable to return for follow-up, patient`s unwillingness to participate, and mental retardation.
According to the possible number of sample size in the BDFP project, the maximum possible number of patients was enrolled. Then, the demographic and clinical information was gathered. Furthermore, actions such as medication interventions, education or referral of patients were conducted as routine when needed.
3.2. Ethical Consideration
The study was approved by the ethical committee of Iran University of Medical Sciences. After collecting the necessary information from the participants and assuring them about the confidentiality of the data, informed consents were taken from the patients as well as from the parents and their immediate relatives. Ethical issues were observed according to the Helsinki declaration 1975, revised in 1983.
3.3. Data Collection Tool
The data were gathered by DAI-10 questionnaire (Table 1) completed for each patient. After completing the questionnaire, comprehensive data about instructions of medication use and medical prescriptions were collected from the patients or heir care givers. These patients were followed every six months. The data gathered from DAI-10 at six months follow-up after discharge from the hospital were compared to the information of medication compliance gathered in the same follow-up (about the use of medications in the last four months) and then were compared to the data of the 12th month. After that, the sensitivity and specificity of the questionnaire was determined. The gold standard to evaluate DAI-10 was defined as the data set taken by the patients or their relatives. To determine predictive validity, the score of DAI-10 at six months follow-up was compared with the medication compliance at the same time and also in the 12th month determined based on (medication possession ratio) MPR. Accordingly, the ratios above 80% were considered as the good compliance (27).
| Question | Answer (True/ False) | Kappa | P Value |
|---|---|---|---|
| 1- For me, the good things about medication outweigh the bad | T/F | 0.630 | < 0.001 |
| 2- I feel strange “doped-up” on medication | T/F | 0.553 | 0.002 |
| 3- I take medications of my own free choice | T/F | 0.769 | < 0.001 |
| 4- Medication make me feel more relaxed | T/F | 0.630 | < 0.001 |
| 5- Medication makes me feel tired and sluggish | T/F | 0.773 | < 0.001 |
| 6- I take medication only when I feel ill | T/F | 0.194 | 0.283 |
| 7- I feel more normal on medication | T/F | 0.368 | 0.041 |
| 8- It is unnatural for my mind and body to be controlled by medications | T/F | 0.478 | 0.05 |
| 9- My thoughts are clear on medication | T/F | 0.552 | 0.02 |
| 10- Taking medication will prevent me from having a breakdown | T/F | 0.783 | < 0.001 |
DAI-10 Questionnaire and Amount of Correlation for Items
3.3.1. The 2nd Part of the Study Was as Follows
To investigate the test-retest reliability, 30 patients with BID in an outpatient clinic in Iran psychiatric hospital were assessed. The patients had been admitted to the hospital for at least once. Five to seven days later, the patients were assessed again. In order to investigate the test-retest reliability, the Spearman-Brown correlation coefficient was assessed and then the reliability of each question with the same question was measured by Cohen’s kappa coefficient (Cohen’s kappa is a common scale, which measures inter-rater agreement for qualitative (categorical) items and the agreement between two raters that each classify N items into C mutually exclusive categories).
3.4. Materials
3.4.1. A 10-Item Drug Attitude Inventory
This is a self-reporting scale developed by Hogan (28). It is translated and validated in many languages (28, 29) and the current questionnaire is derived from the 30-item one after many analyses, but the validity and reliability of the Persian version (DAI-P-10) is not established in Iran yet. The original and the revised versions are translated from the English version by faculty members of Iran University of Medical Sciences; Shabani (corresponding author), and Shariat (14th author). It is currently used in BDPF study (26). This questionnaire is the short-form of the 30-Item DAI, which evaluates both the mental emotions and the patient’s attitude regarding medications. The questionnaire consists of 10 items where answering to the questions indicating a positive attitude to drugs (i e, questions 1, 3, 4, 7, 9, and 10) gets the score +1, and answering to the questions indicating a negative attitude to the drugs (i e, question 2, 5, 6, and 8) gets the score -1 (Table 1). Finally, the total score of the questionnaire is between -10 to +10.
3.4.2. Medication Possession Ratio
This ratio demonstrates the days in which a patient has consumed at least a dose of 80% of its prescribed medicine divided to overall assessment days. This ratio is determined based on the information gathered from the patients and their companion, and if required outpatient documents (27).
The data were analyzed with SPSS version 19. The Spearman correlation coefficient was employed to measure the correlation between the two questionnaires, and Kappa coefficient to determine the reliability of each item. Also, Cronbach’s alpha coefficient was used to assess the internal consistency, and the Spearman correlation coefficient to compare the DAI-P-10 with MPR. In addition, factor analysis was conducted in order to assess the factor validity.
4. Results
The demographic information of both samples is presented in Table 2.
| Variables | Validity Population (N = 83) | Test-Retest Population (N = 30) |
|---|---|---|
| Age, y | ||
| Median | 33 | 38 |
| Range | 18 - 57 | 23 - 62 |
| Disease duration, mo | ||
| Median | 07 | 132 |
| Range | 0 - 34 | 1 - 480 |
| Gender, % | ||
| Male | 63 (64.6) | 18 (60) |
| Educational level | ||
| Illiterate | 49 (56.7) | 0 (0) |
| Under diploma | 27 (32) | 14 (46.7) |
| Diploma | 6 (3.7) | 11 (36.7) |
| Higher education | 0 (0) | 5 (16.7) |
| Marital status | ||
| Single | 32 (39) | 11 (36.7) |
| Married | 36 (43.9) | 16 (53.3) |
| Divorced | 14 (17.1) | 2 (7.6) |
| Widowed | 0 (0) | 1 (3.3) |
| Substance use | ||
| Any substance us | 20 (24.4) | 6 (19.9) |
| Psychiatric comorbidities | 8 (9) | 6 (20) |
| Medications | ||
| Lithium | 32 (39) | 9 (30) |
| Na-valproate | 55 (67.1) | 16 (63.3) |
| Carbamazepine | 3 (3.7) | 1 (3.3) |
| Lamotrigine | 2 (2.2) | 0 (0) |
| Antipsychotic | 75 (91.5) | 25 (83.3) |
Profile of the Studied Persons (Validity and Test-Retest)a
4.1. Reliability Assessment
4.1.1. Test-Retest Reliability
The mean (± SD) score of DAI-10 was 4.6 ± 4.673 for the first test and 5.17 ± 3.896 for the second test. Moreover, the median of DAI-10 was 4 (ranging -6 to +10) for the first test, and 6 (ranging -2 to +10) for the second test. The Wilcoxon sum of ranks showed no significant statistical difference between the two tests (Z = 1.74 and P = 0.283) regarding DAI scores. The Spearman correlation coefficient between the two tests was 0.822 (95% confidence interval (CI): 0.652 - 0.901) (P < 0.001). Furthermore, the consistency related to each item of DAI-P-10 was compared with Kappa statistics in two tests as shown in Table 1. Based on this Table, all the items except for items 6 and 7 had medium to good reliability, but the statistical difference for item 6 was not significant.
4.1.2. Internal Consistency
Based on the results (α = 0.787 and 95% CI: 0.712 - 0.850), removing none of the items can affect the results.
4.2. Validity Assessment
4.2.1. Content Validity
To assess content validity, the values of the content validity index (CVI) were measured. According to the measured values of CVI, based on the eight experts’ viewpoint, the values of CVI for relevance, clarification, and simplicity were 0.86, 0.90, and 0.90, respectively indicating good and acceptable validity of the employed tool.
4.2.2. Concurrent Validity
For this purpose, MPR at the time of assessment was compared with DAI-10 score in the same assessment. The Spearman correlation coefficient at the same time was average and significant for scores of DAI-10 and concurrent MPR (r = 0.676; 95%CI: 0.501 - 0.832, P < 0.0001). Based on MPR ≥ 80%, 70.7% and 29.3% of patients had good and poor compliance, respectively. Based on ROC curve, the cut off point +1 was determined for the DAI-10 questionnaire for the two poor and good compliance groups. Based on the cut off point +1, the sensitivity of DAI-10 was about 82.8%, and its specificity was about 75%. According to this, the positive and negative predictive values of DAI-10 for medication compliance were 88.9% and 64.3%, respectively.
4.2.3. Predictive Validity
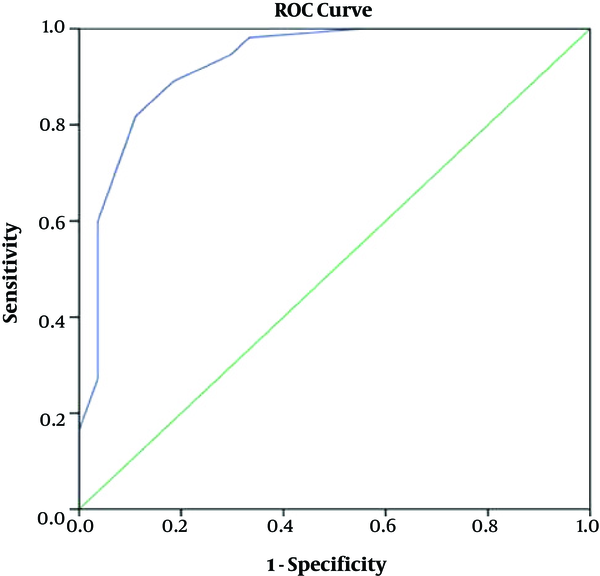
The median of DAI-10 scores was 4 and variation range was -10 to +10, and the median of MPR was 100. The variation ranged 0 to 100. The Spearman correlation coefficient for the scores of DAI-10 and MPR was average six months later and it was significant based on the amount of r (r = 0.663, P ≤ 0.0001). Based on MPR ≥80% and on six months later, the 65.9% of the patients had good compliance and 34.1% had poor compliance. The area under the curve of the ROC curve was 0.926 (P ≤ 0.0001) to determine the cut off point.
shows shows ROC (receiver operating characteristic curve) for DAI-10 score based on MPR.
Table 3 shows sensitivity and specificity of each DAI-10 score in order to predict MPR.
| Specificity | Sensitivity | Score | |
|---|---|---|---|
| 1 | 0 | 1 | -11 |
| 2 | 0.037 | 1 | -9 |
| 3 | 0.225 | 1 | -7 |
| 4 | 0.444 | 1 | -5 |
| 5 | 0.667 | 0.982 | -3 |
| 6 | 0.703 | 0.935 | -1 |
| 7 | 0.815 | 0.891 | 1 |
| 8 | 0.889 | 0.818 | 3 |
| 9 | 0.963 | 0.600 | 5 |
| 10 | 0.963 | 0.273 | 7 |
| 11 | 1 | 0.164 | 9 |
| 12 | 1 | 0.000 | 11 |
Sensitivity and Specificity of DAI-10 to Predict MPR
Based on this the cut off point +1 to separate two good and poor compliance groups the DAI-10 has a sensitivity of 89.1% and a specificity of 81.5% and this point seems a suitable cut off point. Accordingly, the positive predictive value of DAI-10 score for medication compliance was 90.7 % and the negative predictive value was 78.6%.
4.2.4. Factor Validity
The factor analysis was used to measure the index of the factor validity of DAI-10. First, the index of KMO (Kaiser-Meyer-Oklin) was used to determine the adequacy of the sample, and the KMO higher than 0.05 was considered as the statistical measure of sampling adequacy. Moreover, the significance of the Bartlett test of sphericity was considered as the supplemental index.
Based on the formula 5.125/√ (n - 2), 0.576 was considered as the critical value of each item in a factor. However, the reduced number of 0.5 was used in order to include most of the items. Furthermore, to identify the factors, the Eigen value was equal to one. Factor analysis was performed initially without rotation. According to the fact that a number of factors were loaded on several factors, orthogonal analysis with varimax rotation was performed. However, since a significant correlation was observed between the extracted factors and the assumption of the uncorrelated factors was rejected, the factor extraction was redone by the means of oblimin rotation (####) and the Kaiser normalization. In the preliminary evaluation of the factor analysis, the KMO index of sampling adequacy was 0.761, which was an indication of the sample adequacy (P < 0.001; df = 45; Approximate X2 = 194.820); P < 0.001). Due to the lack of adequate component matrix without rotation, the number of unrotated factor analysis was not demonstrated. Without the rotating factors and without taking the critical value of 0.5 into account, at least two items (items 7 and 9) were loaded in two factors and two items (items 6 and 8) were not in any of the factors. With the rotating factors and taking Eigen value >1 into account in order to extract the factors, three factors were extracted from the varimax rotation. The 1st factor with the Eigen value = 3.484, was accounted for 34.840% of variance. The 2nd factor with the Eigen value of 1.254 contributed to 12.539% of variance and the 3rd factor with the Eigen value of 1.056 contributed to 10.563% of variance. In total, the three factors contributed to 57.942% of the total variance. According to the extracted correlation factors, rotation was redone by means of oblimin method, in which, with the approval of the general model, the correlations between the 1st, 2nd, and the 3rd factors were 0.253 and 0.034, respectively. The correlation between the 2nd and 3rd factors was 0.076. In this model, the matrix pattern shown in Table 4, items 1, 2, 4, 5, 7, 9, and10 in the 1st factor, items 6 and 8 in the 2nd factor, and item 3 was loaded on the 3rd factor.
| Item | First Factor | Second Factor | Third Factor |
|---|---|---|---|
| 1 | 0.722 | -0.135 | 0.055 |
| 2 | 0.727 | -0.143 | -0.283 |
| 3 | 0.046 | 0.086 | 0.954 |
| 4 | 0.662 | 0.150 | -0.027 |
| 5 | 0.630 | 0.184 | 0.010 |
| 6 | -0.074 | 0.7898 | 0.060 |
| 7 | 0.734 | 0.177 | 0.195 |
| 8 | 0.087 | 0.779 | -0.148 |
| 9 | 0.527 | 0.381 | 0.083 |
| 10 | 0.771 | -0.125 | 0.061 |
A Matrix for Pattern of Factors Extraction in DAI-10 Index Using Oblimin Rotation
5. Discussion
The current study demonstrated that DAI-10 had appropriate internal consistency and test-retest reliability; although in a separate review of each item, item 6 had no favorable test-retest reliability. This unreliability was likely due to ambiguity of the question. Without considering the patients’ insight, they were asked if they would take medication only when they felt sick. Although the negative answer takes the positive score it might mean the lack of patient’s insight to the disorder. For instance, the patients might be said that they are not ill and they never use medications. The ambiguous nature of the question especially may have been influenced by patients’ perception of the way questions were asked by the examiner. It can be a perceived problem considering that the retest was done through a phone call. Indeed, there was no significant difference after removing this question. In addition, the results revealed a significant relationship between DAI-P-10 scores and the amount of patient’s compliance in six months. This finding can be useful to predict medication compliance and the unique treatment interventions for the people with a negative attitude. These findings can be also helpful to increase medication compliance (24).
The questionnaire is translated and used in several languages. In some of the countries, such as China, Korea, and Spain, the validity and reliability were investigated using different ways, both in terms of the population under study and the method of assessing the validity and reliability of the study (25, 30, 31).
In the current study, the score of DAI-10 had a significant relationship with medication compliance of patients at the time of investigation in which the finding was consistent with those of other studies (30, 32, 33). Moreover, many other studies showed a positive correlation between medication adherences and positive attitude towards medication (34, 35). Furthermore, unlike the current study, Kikkert in Europe showed that neither DAI-10 nor other tools such as medication adherence questionnaire and compliance rating scale could predict the outcome of disease in the patients with schizophrenia, such as the time of recurrence, the number of hospitalizations, and the number of relapses in 12 months (36), contrary to the results of the current study. Perhaps, one of the reasons can be the different nature of schizophrenia and bipolar disorder. In addition to differences in the type of the study and the type of disease, the difference in the duration of the study may also explain the difference in the results. In the current study, the internal consistency was equivalent to 0.758 in which these findings were consistent with the other researches conducted in the other countries (25, 37, 38). In another study, the test-retest reliability of DAI-10 in the patients with schizophrenia was reported high, which was consistent with the current study results (18). The overall results of the test-retest reliability were consistent with those of the other studies, but it is recommended that the reliability of each of the questions be measured with those of the other studies individually and possible causes of the low reliability of the item 6 be investigated. Three factors were obtained in the factor analysis of the study. The 1st factor included items 1, 2, 4, 5, 7, 9, and 10; the 2nd factor included items 6 and 8; and finally, the 3rd factor included item 3. Also, in the study by Nielsen three factors were obtained. The 1st factor included items 1, 4, 7, 9, and 10; the 2nd factor included items 5 and 8; and the 3rd factor included items 2, 3, and 6 (19). The 1st factor of Nielsen’s study includes all factors of the current study, and his/her 2nd and 3rd factors were common to items 8 and 3 of the current study, respectively. In the current study, it seems that subjective items were placed in the 1st factor and the objective ones in the other factors. As it is clear, item 6 was an exception and this can be justified by the previous results and the uncertainty of items.
In general, the DAI-10 has appropriate internal consistency, the test-retest reliability, and the predictive validity for the patients with bipolar disorder type I. But in spite of that, further studies are required to generalize the data to other groups such as the patients with BIID. Due to the limitations of the current study to investigate the predictive validity, the poverty of other similar studies, and the difference in the results in comparison with other studies, it is recommended that further investigations on the validity issue be conducted. For example, its relationship with treatment outcome or recurrence in the future (as a sign of non-adherence) can be evaluated, and even to investigate more accurately, evaluating serum levels of medications such as sodium valproate and lithium can be conducted instead of MPR. Furthermore, in the future studies other tools such as “scale to assess unawareness to mental disorder (SUMD)” in addition to DAI-10 can be used in order to evaluate attitude, and also it is recommended to allot more time (more than six months) to determine the predictive validity. Among other limitations of the current study, the following might be noted: As it was not necessary for the patients to revisit for the medical purposes after a week, and as it was amoral to ask them to return just for doing the survey, alongside with the commuting problems as well as the extra costs in investigating reliability of the test-retest for second time, the test was conducted by a phone call. The authors also used some reports gathered from patients and their companions, while investigating the validity of the questionnaire, to calculate the MPR index, but these reports may lack the accuracy due to some reasons such as retrospective questions, which caused difficulty to remember the exact dose, number of days of medication treatment, and the number of days of the prescription dose. Another limitation of the current study was the investigation of validity and reliability in different populations due to technical limitations.
5.1. Conclusions
Results of the current study showed that DAI-10 questionnaire in patients with bipolar disorder was a tool with relatively good reliability and could predict medication compliance in the future. Despite the limitations of the current study and incompatibility of some of the findings with similar studies, more studies are needed to prove the findings of the current study; it is recommended that the efficiency of this tool be separately investigated to predict the use of the drugs. Since attitude is only one part of the compliance and as these two are not necessarily connected with one another, it is suggested that DAI-10 be evaluated by other tools such as (SUMD) in order to obtain more accuracy to evaluate the utility of DAI-10 to predict the use of medication by eliminating the effect of insight about the disease.