1. Context
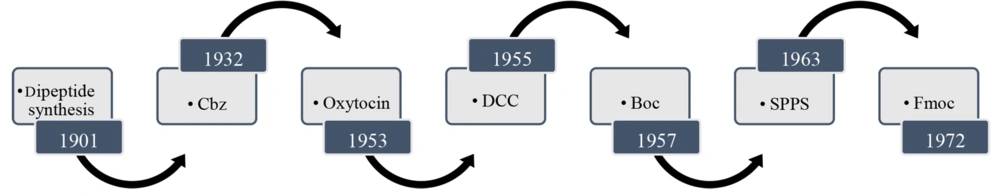
In 1881, Curtius synthesized the first N-protected dipeptides containing blocked amino groups like acyl, acetyl, or benzoyl (1). Although, at that time, there was no particular way to deprotect the nitrogen atom without breaking the peptide bond. In 1901, Fischer and Fourneau developed the Gly-Gly dipeptide (2). This is why Emil Fischer is called the "founding father" of peptide chemistry. His significant impact on peptide development was using α-chloro acid chloride to condense with an amino ester and then replacing the Cl group with an amine to obtain a peptide. Nonetheless, this synthesis gives racemate peptides (3). Through this method, dipeptides, and later tripeptides and polypeptides, were prepared.
It was in the midst of the progress of this discovery that World War I began, and its progress was postponed for fifty years. However, Bergmann and Zervas found that the Cbz-protecting group kept the configuration of the chiral center and prevented racemization during carbamate formation, which is a good replacement for N-acyl- or N-benzoyl-protected amino acids (4). This was a revolutionary discovery in the synthesis of peptides. After World War II, du Vigneaud isolated and identified the amino acid sequence of the polypeptide hormone 'oxytocin' and then synthesized it (5-7). Because of his discovery, du Vigneaud was awarded a Noble Prize in 1955.
It is necessary to protect the amine groups and deprotect them in a timely manner to prevent the production of by-products and self-condensation of amino acids. As mentioned above, Cbz-protected amines can be deprotected through hydrogenolysis using Pd-charcoal or HBr (8). The need for a cheaper protection-deprotection method led to the discovery of the tert-butyloxycarbonyl (Boc) group in 1957 (9). The conventional methods for deprotection of N-Boc amines are: (1) heating the solution in the presence of HCl (10); and (2) dissolving the protected amine in a mixture of HCl/TFA at room temperature (11). Once the problem of protecting amines was resolved, the efficient formation of peptide bonds was another challenge. Meanwhile, Sheehan and Hess introduced a new method for the efficient formation of peptide bonds using ‘dicyclohexyl carbodiimide’ (DCC) as a coupling reagent (12). In 1972, Carpino and Han found a new way of amine protection using the 9-fluorenylmethoxycarbonyl protecting group (Fmoc). However, deprotecting nitrogen atoms containing the Fmoc group is rapidly performed in the presence of secondary amines, especially piperidine, in DMF (13). This development is summarized in Figure 1.
Despite all these advances, until the early 1960s, the synthesis of peptides was so difficult that a project took one to two years until Merrifield's innovation resulted in a great revolution in peptide synthesis (14). In the solid phase peptide synthesis (SPPS) method, an N-protected amino acid is bound to a resin or other substrates from its C-terminus via an amide or an ester bond. After amine deprotection, it is reacted with the carbonyl group of the subsequent N-protected amino acid. This cycle is repeatable to achieve the favorite peptide chain. Finally, the formed peptide is cleaved from the resin (15, 16). Soon after, various types of beads were introduced and commercialized, like polystyrenes, BHA, and Wang resins (17).
Therefore, modified and improved synthetic peptides have emerged on the market. When Novartis Pharmaceuticals introduced lypressin, an antidiuretic hormone and analogue of vasopressin to regulate the tonicity of body fluids, in the 1970s, the therapeutic peptide business was born. Most physiological processes are regulated by peptides, and they may act as endocrine and paracrine signals, neurotransmitters, or growth factors. Although peptides do not have all of the desirable characteristics of an ideal medicine, they have properties like high affinity, specificity, and the capability to stay on target longer due to their size. Moreover, as therapeutic candidates, peptides have a predominantly specific activity compared to small molecules because peptides are simply destroyed in the human body. According to these properties, peptides are becoming more popular than other medicines for certain diseases, disorders, and infections, where the direct introduction of therapeutic peptides is desirable. Furthermore, peptides generally have low adverse effects and have become appealing medication design choices (18-21).
In 2015 and 2020, the market for therapeutic peptides was valued at $17,568.0 and 28,510.60 million, respectively, which is expected to increase to $51,360.30 million in 2026 (22). Now, eighty FDA-approved therapeutic peptides are on the market, and hundreds of peptides are in clinical and preclinical trials (23). As a result, peptide and protein-based pharmaceuticals are rapidly becoming an important class of medications. They are likely to replace many existing small-molecule pharmaceuticals in the very near future.
2. Peptide Synthesis
There are two conventional methods for peptide synthesis, the liquid (solution) phase peptide synthetic approach and the solid phase peptide synthetic approach. This increases the need to pay attention to developing waste reduction methods. Here below, we briefly explain these methods.
2.1. Liquid Phase Peptide Synthesis
This is the method that Fischer used to synthesize the first dipeptide Gly-Gly. Through the protection of the carboxylic acid group of the first amino acid and the amino group of the second one, a peptide bond is formed between the free amine and carboxyl groups. This process is repeated to achieve the desired sequence and is finalized by the selective deprotection of the two protecting groups, which gives the free peptide. This classical methodology is not preferably suited to the synthesis of long-chain peptides due to their low solubility and difficult purification. Isolation and purification of the intermediates are the major drawbacks of this approach. However, some di-, tri-, and tetra-peptides are prepared using the liquid phase method (24, 25).
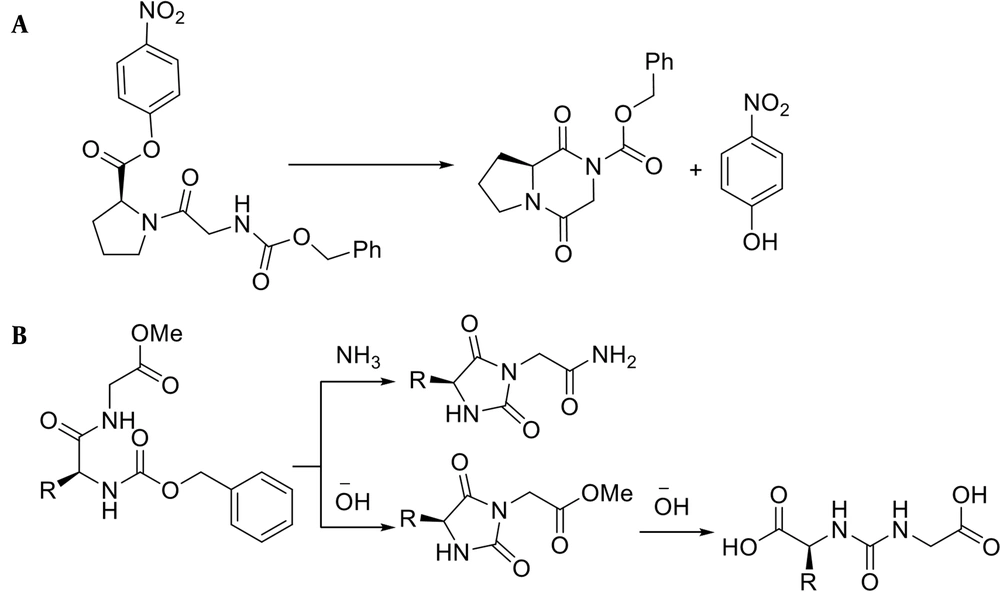
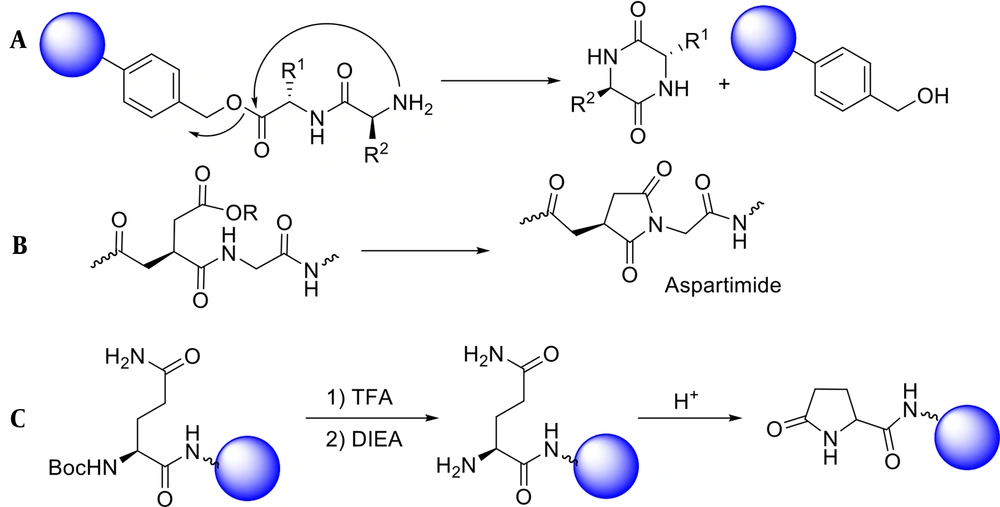
In the case of dipeptide synthesis, especially Gly-Pro, the protected carboxyl neighboring the α-amino group has a high tendency to be cyclized to form diketopiperazine (Figure 2A) (26). Moreover, peptides comprising Gly and benzyloxycarbonyl, as a protecting amine group, can be cyclized under basic conditions to give hydantoin (Figure 2B) (27).
2.2. Solid Phase Peptide Synthesis
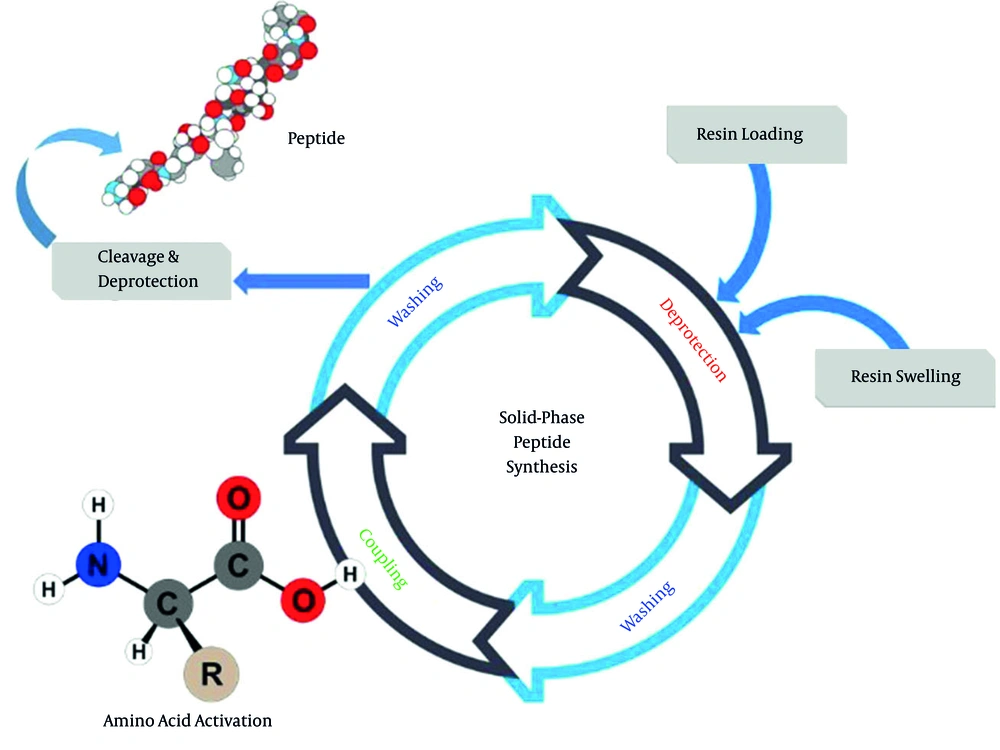
The summary of solid phase peptide synthesis (SPPS) is shown in Figure 3. This method was developed by Merrifield (15, 28). The SPPS concept is based on the attachment of the first amino acid to a swelled solid polymer/resin by a covalent bond. The free sites of the loaded bead must be capped to avoid undesired reactions. After washing, deprotection of the loaded amino acid is performed. The coupling reaction with the next protected amino acid is accomplished after washing. Again, washing, deprotection, washing, and coupling reaction are repeated to assemble the desired peptide sequence. The synthesis is finalized by deprotection and detachment from the bead using TFA (29).
Since the assembling peptide chain is firmly attached to an insoluble but swollen bead, it can be easily filtered off and washed to remove the reagents and by-products. Consequently, the intermediate peptides can be completely purified by dissolving away the impurities, not by recrystallization. Therefore, this procedure is straightforward and fast. The main challenge with SPPS is using large amounts of expensive beads, protected amino acids, reagents, and solvents as they raise the overall manufacturing cost (30). However, informed by this knowledge, chemists can now manufacture their favorite peptide sequences.
Although the SPPS approach is the most widely used for peptide synthesis, it is not without flaws. Even when all instructions are correctly followed, depending on the type of amino acid used in the peptide sequence, or the type of synthetic strategy of Boc/Bzl or Fmoc/t-Bu, various side reactions may occur (31). Since an undesirable cyclization reaction is likely to occur before the binding of the third amino acid, the synthesis of carbon-terminated peptides requires a particular approach. This cyclization reaction results from the intramolecular addition of the free amine group on the di-peptide-resin to the peptide-resin ester bond, forming a cyclic peptide, diketopiperazine (Figure 4A) (32).
Cyclization of aspartic acid and formation of aspartimide are other side reactions in SPPS. Fortunately, these side reactions occur when only Asp-Ser, Asp-Ala, and Asp-Gly sequences are present (Figure 4B) (33). In amino-terminus peptides, intramolecular cyclization of the glutamine in the presence of acid leads to the formation of pyroglutamic acid. This side reaction only occurs in Boc/Bzl approaches (Figure 4C). It is better to use HCl for Boc-deprotection to prevent such unwanted cyclization (34, 35).
The use of HF to detach peptides comprising His(Boc) from the resin surface leads to forming formaldehyde. Therefore, the side chains of sensitive amino acids can be methylated in the presence of the formed formaldehyde (36). However, using resorcinol as an absorber of formaldehyde may help avoid side reactions (37). Furthermore, some peptides, especially those containing Met and Asp-Pro, are sensitive to HF, resulting in the fragmentation of their sequences (38).
3. Chemical Wastes of Peptide Synthesis
As already mentioned, SPPS is one of the most popular peptide synthesis methods. Let's take a glimpse at the process. From the beginning, we will notice that most of the used chemical substances pollute the environment and increase the cost of peptide production.
3.1. Resin Swelling
First, various resins are employed in the SPPS technique, among which polystyrene (PS), pure cross-linked polyethylene glycol (PEG), and PS-functionalized PEG are the most popular resins. The PS resins are cheaper and suitable for producing short- to medium-length peptides. The polystyrene resin, with an initial loading of up to 1.5 mmol g-1, is cross-linked with divinylbenzene and is compatible with DCM and DMF, although it is not compatible with water. In contrast, PEG-based resins are more expensive and are frequently used in the synthesis of medium- and long-length peptides, as well as peptides comprising challenging sequences. The PEG-PS resins, with an initial loading of up to 0.6 mmol g-1, are compatible with most solvents, such as PEG, DCM, DMF, THF, and even EtOH and water. Therefore, PEG-based beads are good candidates for use under green conditions, but they need higher amounts of solvent per mass to be swelled (39). Aside from considerable solvent consumption and, in many cases, the use of hazardous and environmentally contaminating solvents, recycling these resins has long been a matter of concern.
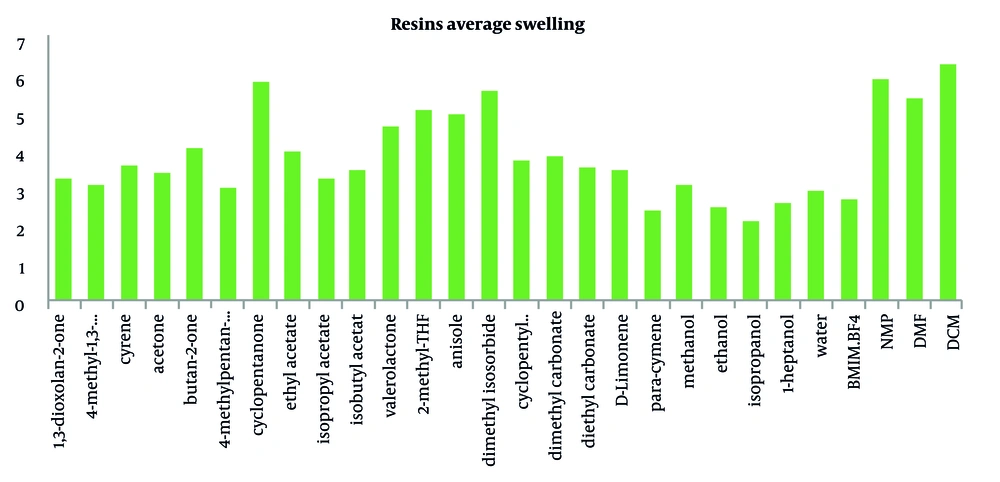
North et al. investigated the swelling factor of relevant resins used for SPPS in 25 solvents, including DMF, DCM, and NMP, to find the best green solvent (40). Nine common resins (Merrifield, Jandajel, Paramax, TentaGel, ArgoGel, HypoGel, NovaGel, ChemMatrix, and SpheriTide) were chosen to test in 25 green solvents for analyzing resin swelling ability in green solvents. The average swelling of experimented resins in these solvents is demonstrated in Figure 5. The swelling of each resin in solvents was evaluated using Griffith et al.'s method (41), and solvents covered a wide range of polarities. ChemMatrix demonstrated the best result with average swelling < 5 mLg-1; for the solvents, DCM and DMF demonstrated the best results. However, in green solvents, cyclopentanone represented promising results. It showed slightly lower swelling properties than DCM but had a better average than DMF and NMP.
Despite little research focusing on its applicability in SPPS, the poly-ε-lysine resin can be used as an appropriate bead in peptide synthesis (42, 43). The biodegradable poly-ε-lysine resin is prepared by microbial fermentation and is widely used in food industries, highly water absorbable hydrogels, anticancer formulations, biodegradable fibers, drug carriers, and agriculture (44).
Besides, modifying resin types or using biodegradable ones can reduce the waste from the beads. Colzani et al. functionalized the biodegradable poly-lactide-co-glicolide (PLGA) with small peptides to be studied as a drug delivery system (45). This polymer is widely employed for encapsulation or formulation of peptides (46-49). However, the poly-lactide-co-glicolide may act as a green bead in peptide synthesis.
3.2. The Coupling Reagents
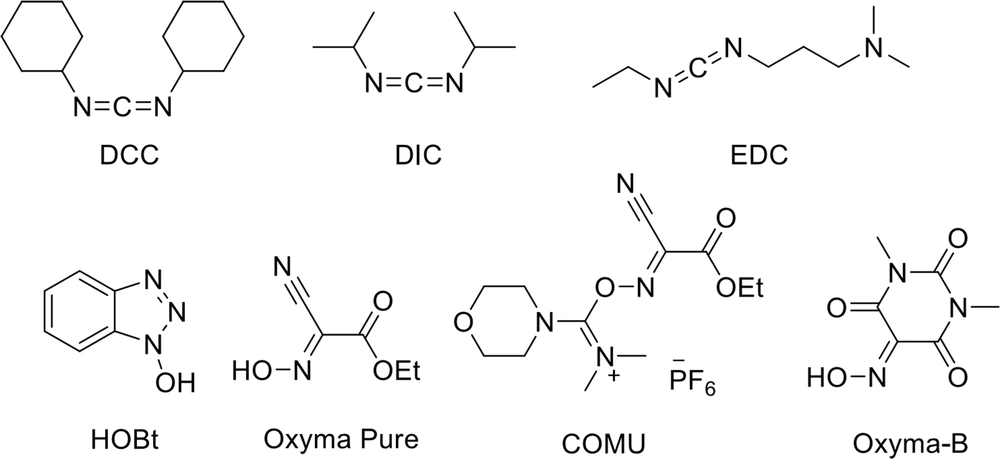
Unfortunately, forming an amide bond is not easy, and coupling reagents are used to increase its efficiency. Carbodiimide compounds, such as N,N′-dicyclohexylcarbodiimide (DCC), N,N′-diisopropylcarbodiimide (DIC), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), and oxime or N-hydroxy compounds, like 1-hydroxy-1H-benzotriazole hydrate (HOBt), COMU, Oxyma Pure, Oxyma-B, (Figure 6) and their derivatives, are commonly used as coupling reagents in the formation of peptide bonds. Among them, COMU and DIC are the most expensive reagents, while DCC and Oxyma Pure are the cheapest ones. These coupling reagents can increase the efficiency of peptide bond formation and prevent amino acid racemization. However, the formation of water-soluble urea is one of the biggest problems in their use (50).
There is no doubt that benzotriazoles like HOBt and their derivatives are explosive, making handling, preserving, or scaling-up difficult (51). In 2008, Hayes et al. identified DIC and DCC as irritants and contact sensitizers (52). Although Oxyma, as an alternative to HOBt, is commonly used as a mixture with a carbodiimide (53), McFarland et al. reported the release of hydrogen cyanide from the reaction of Oxyma Pure and DIC in DMF at room temperature (54). Using an Oxyma and COMU mixture could solve this problem (55), but COMU is not stable in DMF and is the least preferred reagent in SPPS (56, 57). In general, these coupling reagents display an inferior atom economy.
Lastly, we need to develop and find alternative greener methods for forming peptide bonds and improving atom economy and compatibility of coupling reagents with various solvents, especially green ones (58).
3.3. The Protected Amino Acids
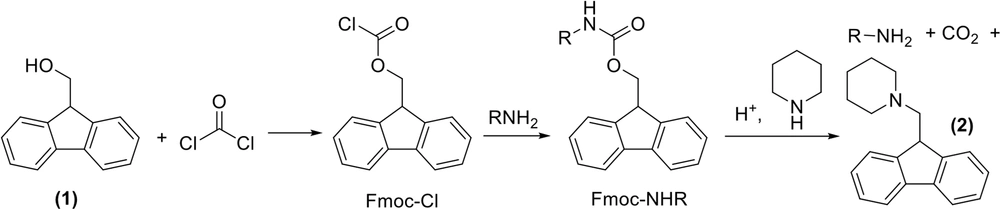
The important hidden aspect of the protected amino acids is that the protection process stands on the many environmental contaminations. Fluorenylmethyloxycarbonyl chloride (Fmoc-Cl) is utilized in the protection of amine groups. It is manufactured by reacting 9-fluorenylmethanol 1 with phosgene (Figure 7) (13). The latter is one of the most poisonous chemicals and was the cause of 85,000 deaths during World War I (59). Furthermore, considering the preparation of precursors, various solvents are used during the synthesis, purification, and recrystallization of all these compounds. Besides, 20% piperidine in dimethylformamide is the way of Fmoc deprotection, both of which are not sustainable. However, in this case, γ-valerolactone is a green alternative to DMF (60). As illustrated in Figure 7, compound 2 is the by-product of the Fmoc deprotection and must be considered as contamination because it is useless and is deposed (61).
In some cases, di-tert-butyl dicarbonate (Boc2O) is employed to protect those amine groups that must not be bonded with a carboxyl. Correspondingly, phosgene is one of the precursors of Boc2O production. The Boc-protected amines are finally deprotected using a solid acid, such as TFA or HCl in methanol. TFA is almost non-degradable in the environment and is toxic to aquatic life (62, 63). The hazardous nature of methanol is evident.
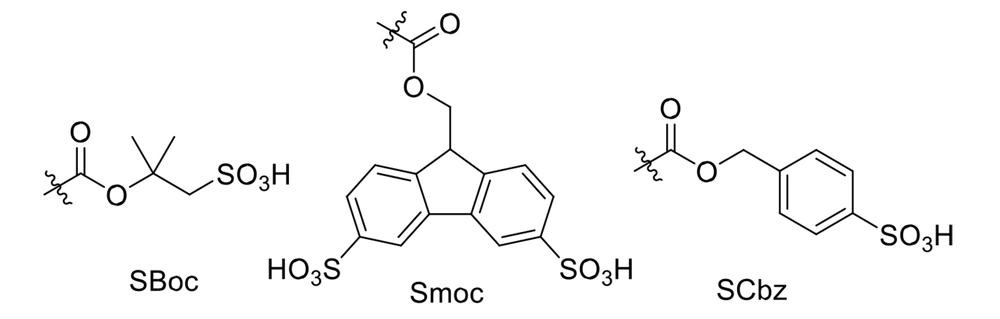
So far, various protecting groups have been designed but never widely or industrially used (64-66). For example, Carpino et al. designed and utilized protecting group 1,1-dioxobenzo[b]thiophene-2-ylmethyloxy carbonyl, abbreviated as Bsmoc, in the aquatic phase peptide synthesis (APPS). They found a mechanism for the deprotection process leading to the efficient removal of Bsmoc (67). It has been used in some academic research, while its industrial usage is costly (68). Knauer et al. could commercialize new Boc-, Fmoc-, and Cbz-analogs (SBoc, Smoc, and SCbz, respectively) comprising the sulfonic acid group (Figure 8). These protecting groups are compatible with water and act efficiently in the APPS technique, leading to the elimination of toxic solvents. The deprotection is performed using sulfoacetic acid or sulfobenzoic acid, showing the sustainability of this newborn achievement (69).
3.4. The Solvents
As mentioned in the introduction section, the SPPS contains a repetitive sequence of coupling, washing, deprotection, and washing. Therefore, large amounts of solvents, mostly DCM, THF, and DMF, are used during peptide synthesis. Although recycling these solvents reduces environmental contamination, the use of low boiling point DCM always has its drawbacks, especially for the ozone layer.
Solvents like ketones, esters, ethers, and alcohols can be used in resin swelling. Among ketones, including 1,3-dioxolan-2-one, dihydrolevoglucosenone (Cyrene), 2-butanone, 4-methyl-2-pentanone, and cyclopentanone, the latter shows the highest swelling that is comparable to DCM (70). Therefore, cyclopentanone is a good alternative to DCM in peptide synthesis. Alcohols and phenols show the lowest swelling effect, while NMP, DMF, DCM, esters, and ethers are the best swelling solvents. Among them, γ-valerolactone is a green solvent that can be used as an alternative to DMF that shows a moderately high swelling effect and can dissolve amino acids and reagents (71). PolarClean is a by-product of Nylon-66 manufacturing. Kumar et al. realized that it could highly dissolve all Fmoc-amino acids and critical coupling reagents and additives. PolarClean is a valuable solvent for swelling polystyrene and ChemMatrix resins (72).
4. Sustainable Methods
Isidro-Llobet et al. published a comprehensive review discussing the sustainability of peptide synthesis in scale-up methods (73). They presented novel green industrial methods, including peptide synthesis in flow (74, 75), greener tag-assisted liquid phase peptide synthesis (76, 77), nanofiltration (78), membrane-enhanced peptide synthesis (79, 80), enzymatic peptide synthesis (81, 82), and mechanochemistry (83, 84). Moreover, Martin et al. thoroughly reviewed the green approaches for SPPS (85).
4.1. Flow Chemistry in Peptide Synthesis
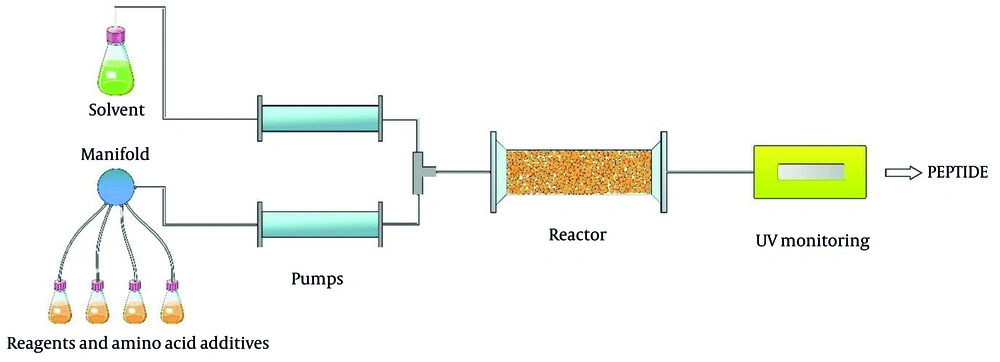
Due to the green features of flow chemistry, it has become one of the most used methods for future peptide production. The benefits, including safety, space-saving ability (reaction space < 1 mm), and production capacity, make flow chemistry a useful method for peptide synthesis. Continuous flow begins with two or more feeds. For instance, reagents and reactants are pumped into a micro reactor or tube. Then, they are mixed and react with each other. Due to the small size of the chamber and shafts in the reactor, synthesis can be accomplished by using a slight amount of reagents (Figure 9). Thus, this technique reduces the use of solvent and reagents, making it a cost-effective method. Moreover, it facilitates heat transfer and reduces reaction time (86, 87).
In this way, researchers have done several studies yielding significant results. Fuse and co-workers showed that applying the continuous flow method significantly reduced reaction time and made strongly activated amino acids like acid anhydrides perform coupling efficiently. Due to low and controlled volume flow, the least racemization is observed. The solvents used in this process were DMF and MeCN, and tetra to dipeptides were synthesized with good yields. They have also demonstrated that microflow reactors can synthesize amino acids such as N-carboxy anhydrides (NCAs) with the aid of triphosgene (88-91).
NCAs are an effective method for peptide synthesis, and Jolley and co-workers demonstrated that NCAs played a significant role in large-scale peptide synthesis. In addition, it was shown that flow peptide synthesis was suitable for short peptide synthesis and those synthesis methods not common in batch methods (92).
Pentelute et al. demonstrated the flow-based SPPS method as an approach that facilitates the synthesis process and saves time. The automatic control option allows this method to incorporate each amino acid residue every 48 seconds. Low reaction volume allows continuous delivery of heated solvent, and a UV detector monitors the whole process. This method was employed to synthesize several peptides, and the same result as a batch traditional method was obtained. It was demonstrated that SPPS could be a rapid process utilizing continuous flow and obtained faster results than microwave-assisted peptide synthesis. Drowning sealed vessel into a temperature bath provided solvent with desired heat in contact with resins (93).
In their later study, Pentelute and co-workers surveyed an automated, flow-based method for solid-phase synthesis for polypeptides. The amide bond was formed in 7 seconds, and the total synthesis took about 40 seconds per amino acid residue. Crude peptide purities and isolated yields were comparable to standard-batch solid-phase peptide synthesis.
Overall, it is believed that continuous flow peptide synthesis can be applied for large-scale peptide production in the near future if the proper requirements are provided. One of the flow methods gaining attention is automated fast-flow peptide synthesis (AFPS) provided by Pentelute et al. which is represented as an approach more adaptable to large-scale peptide synthesis and supply chains. The first description of AFPS was reported in 2014 (94). Furthermore, in their later article published in a scientific journal, the highly efficient usage of AFPS for synthesizing large peptides up to 164 amino acids long over 327 consecutive reactions was reported (95). Automated fast-flow peptide synthesis was utilized to synthesize nine different peptides from 86 to 164 amino acids in only 6.5 hours maximum. There are still some problems, such as reactor capacity that is only up to 0.49 mmol g-1, which could be a big drawback for synthesis on a large scale. Another subject that makes this method not the best choice is the usage of hazardous solvents; the development of AFPS was based on DMF, which should be replaced to reach a sustainable approach. Hence, by finding a greener alternative for DMF, this method can be one of the most beneficial and sustainable approaches for future peptide synthesis.
4.2. Microwave Irradiation in Peptide Synthesis
Microwave-assisted peptide synthesis is commonly used for two main reasons: heating the solvent and the reaction mixture and overcoming the solubility of the protected amino acids in organic solvents such as DMF and rapid coupling process. The result is process time reduction and total cost reduction of peptide production. The difference between conventional heating and microwave irradiation method is that the latter heats the reaction mixture directly. In contrast, the former first warms up the containing vessel and then transfers heat to the mixture. Microwave irradiation can be used in solution-phase peptide synthesis, solid-phase peptide synthesis, and even peptide synthesis in water (96, 97). It is noteworthy that microwave irradiation is a proper technique to remove Fmoc protecting agent (98). CEM Corporation was founded based on microwave drying concepts in 1978 and today is a leading company in producing microwave peptide synthesizers. An automated peptide synthesizer uses Fmoc-protected amino acids as reactants and microwave irradiation as the energy source to promote the reaction. In this way, reactions that take hours or even days to complete can be performed in minutes.
Another advantage of microwave-assisted peptide synthesis is the privilege of using green solvents instead of prevalent solvents like DMF. For instance, Albericio et al. investigated using GVL as a solvent with microwave irradiation (99). In addition, for peptide synthesis in solution-phase, Jain et al. surveyed MAOS in neat water (100). Moreover, it was reported as an environmentally friendly, free racemization method that could be beneficial for bio peptide synthesis. Also, for SPPS, Albericio et al. investigated using water for coupling and deprotection steps with microwave irradiation combined with Boc-protected amino acids and numerous coupling reagents (101).
4.3. Membrane-Enhanced Peptide Synthesis
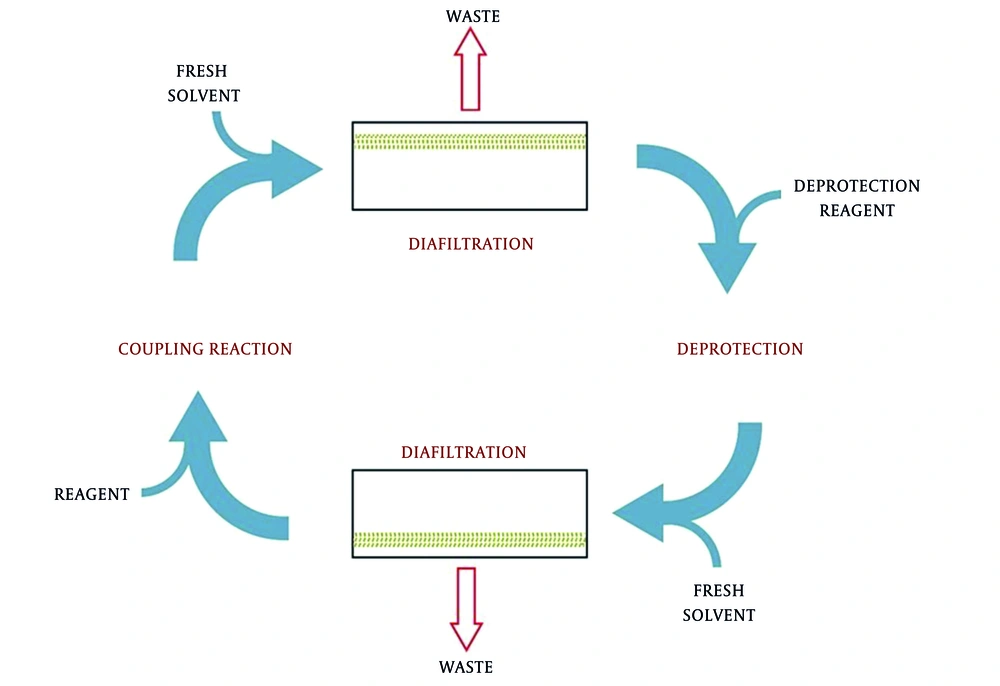
One of these recently developed methods utilizes membrane for peptide synthesis. This method, called membrane-enhanced peptide synthesis (MEPS), combines solution-phase peptide synthesis and organic solvent nanofiltration. Thus, MEPS benefits the advantages of solution-phase peptide synthesis and organic solvent nanofiltration to alleviate the problems facing SPPS. In this method, the peptide chain is assembled through coupling reaction, following by washing step to remove the excess reagents by passing the solvent through the diafiltration membrane. Then, the deprotection step is performed by adding the deprotection reagents and passing again through the diafiltration unit to remove residues. The privilege obtained from this method is that there is no need for changing solvent or adding new solvent, and in addition, this process is easy to scale up beyond kilogram for mass-product in facilities (80, 102, 103). The problem facing MEPS was that the star-shaped molecules could pass through pores of ultrafiltration. Using globular polymers instead of linear PEG was recommended to solve this problem (Figure 10) (104).
In their publication, Todorovic and Perrin discoursed green catalytic approaches in forming the amide bond (56). Much progress has been made in direct amidation, starting from carboxylic acids and amines. Some catalytic systems promote and accelerate the amidation reaction, including Ti and Zr transition metals (105, 106) and organoboron catalysts (107-113). There are also indirect amidation methods in which an ester is treated with an amine in the presence of a catalyst, such as Ni (114-116), Nb2O5 (117, 118), tantalum alkoxide (119), and lanthanide complexes (120). These approaches are inspiring in terms of greening the peptide synthesis and reducing its wastes.
4.4. Native Chemical Ligation Peptide Synthesis
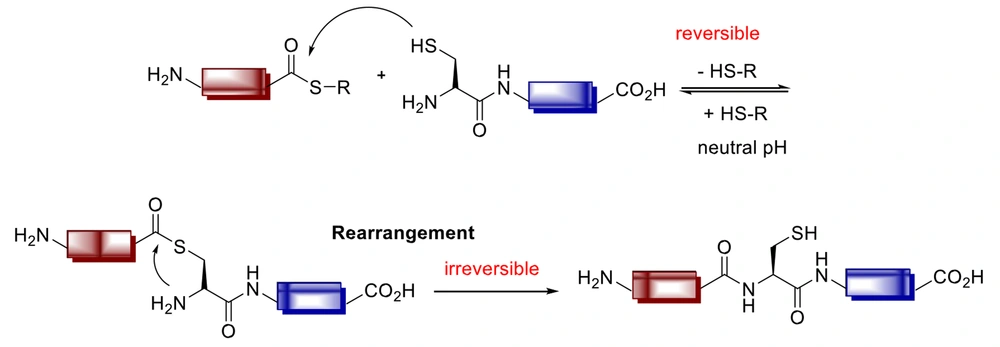
The prospect of native chemical ligation (NCL) was offered to react unprotected thioesters with N-terminal Cys peptides in 1994 by Kent et al. (Figure 11) (121).
The chemoselective ligation of unprotected peptides and proteins has drawn particular attention in peptide and protein chemistry. The solid-phase peptide synthesis is unsuitable for peptides with more than 50 amino acids or large peptides. Thus, investigations have focused on developing chemoselective ligation and modification strategies to link synthetic peptides to larger synthetic and biologically macromolecules (122, 123). In recent years, there have been promising developments for this method, with Staudinger ligation being one of the most important ones (124).
It was demonstrated that ligation synthesis had significantly improved for producing complex and large peptides and APIs. In addition, it has to be noted that this approach is considered a green approach for peptide production. Hopefully, this method will play a vital role in peptide production on a large scale in the near future.
4.5. Novel Technology in Peptide Synthesis
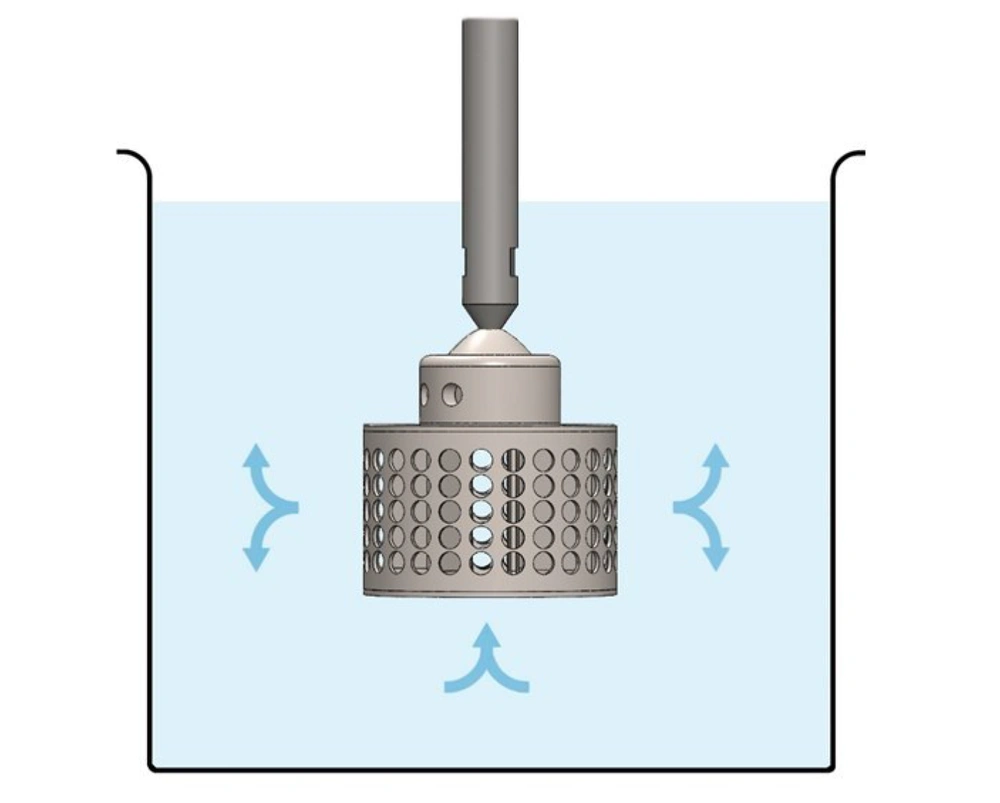
MIT University reported a novel technology that offers fast peptide synthesis. This system can make a dipeptide in 37 seconds and can generate peptides comprising about 60 amino acids in just one hour. This research investigated the capability of rotating bed reactors (RBRs) produced by SpinChem. An RBR (Figure 12) can enhance the ratio of solid-phase to liquid, resulting in less solvent consumption and minimizing the wastes for peptide synthesis. The efficiency of this system was measured by ionic adsorption at the lab and commercial scales, demonstrating approximately similar results. At the lab scale, the decrease of ions was on average 86.5% after 15 seconds, and at the industrial scale, ion reduction was 92.9% after passing 20 seconds. Moreover, it was observed that solvent consumption was reduced by about 82% using RBRs compared with relevant approaches of SPPS (125). This method is cost-effective for peptide synthesis, even in high-scale processes. It also brings peptide synthesis one step closer to the more sustainable and environmentally friendlier zone. This method shows that using new technologies can saves time and is more cost-effective.
Finally, refer to Table 1 to better comprehend the advantages and disadvantages of the various sustainable methods for peptide synthesis.
| Methods | Advantages | Disadvantages |
|---|---|---|
| Continuous-flow chemistry | (1) Safety, (2) Least solvent consumption, (3) Compact and space saving, (4) Time saving | (1) Low reactor capacity, (2) Consumes DMF as the main solvent |
| Microwave irradiation | (1) Intensive reduction of reaction duration, (2) Uniform and direct heat transfer, (3) Adaptable with green solvents, (4) Adjustable with novel methods | (1) Systems with microwave irradiation might be expensive, (2) Adjustable only for lab-scale synthesis |
| Membrane-enhanced peptide synthesis | (1) Same solvent can be used repetitively in the cycle, (2) Ease in scale-up | (1) Some molecules and components can pass through membrane pores |
| Chemical ligation peptide synthesis | (1) Suitable for intricate and large peptide synthesis | (1) More investigation and development is required to turn this method to a practical one for large-scale peptide synthesis |
| Rotating bed reactor (RBR) | (1) Intensively reduces solvent consumption, (2) Time efficient method, (3) Adaptable to larger scales |
5. Conclusions
In this review, we showed that therapeutic peptides were alternatives to other chemical medicines due to their lower side effects. Therefore, peptide-based pharmaceuticals have been expanded in the recent decade. However, there are concerning issues as many harmful wastes are produced during peptide synthesis. The used resins, solvents, the protecting groups of amino acids, and coupling agents produce waste that may be unrecyclable. The design of sulfonic acid functionalized protecting groups opened a window to develop water-based methods of peptide synthesis that solve many problems. In addition, the development of methodologies for the formation of amide bonds may help improve the sustainability of peptide synthesis.