1. Background
Bacterial keratitis is a major cause of visual deficiency in developed and developing countries. As an aggressive ocular infection, bacterial keratitis should be managed by rapid and effective treatment; otherwise, it can cause corneal perforation and blindness (1, 2). Eye injuries and contact lenses are critical risk factors for bacterial keratitis (3). The most frequent bacterium in bacterial keratitis related to contact lens wear is Pseudomonas aeruginosa (Gram-negative) and not related to contact lens cases is Staphylococcus aureus (Gram-positive) (4).
It is exceptionally vital to begin the immediate treatment of bacterial keratitis with a broad-spectrum antibiotic that is active against both Gram-positive (G+) and Gram-negative (G-) bacteria. Topical fluoroquinolones are prescribed as the first-line antibiotics for treating bacterial keratitis (5). The main concern about the treatment of this bacterial infection is emerging antimicrobial resistance. Staphylococcus aureus and P. aeruginosa are ESKAPE pathogens that utilize various antibiotic resistance mechanisms (6). The World Health Organization has identified S. aureus and P. aeruginosa as high and critical priority targets for the development of new antibiotics (7).
Antimicrobial peptides (AMPs), as a part of the innate immune system, comprise a large group of molecules that are found in most living organisms. These cationic peptides, usually with a charge between +2 to +6, are active against G+ and G- bacteria (8). The principal mechanism of action for fast-killing pathogens is an association of AMPs with cell membranes followed by a disruptive effect (9). LL-37 presents in leukocytes and is also secreted by corneal epithelial cells (10). LL-37, as a natural AMP, is active against G+ and G- bacteria; therefore, its structure is a conventional initial model for the design and discovery of various AMPs (11).
Natural AMPs have restrictions to be used as antibiotics for humans, including toxicity, high cost of production, low half-life or stability, and the lack of selectivity to prokaryotic cells. As a result, the discovery of novel AMPs is a critical need to overcome the above-mentioned restrictions and achieve a smaller skeleton with the maximum antimicrobial activity and minimum toxicity (12). For the enhancement of the stability of AMPs against proteases, various strategies have been considered, highlighting peptide dimerization (13, 14). Moreover, the dimeric forms of the selected bioactive peptide sequences have revealed pharmaceutical advantages, such as increasing antimicrobial potency, solubility, and resistance to proteases (15-18). Using in silico methods based on machine learning algorithms is profitable for AMPs’ design. Machine learning algorithms and random optimization methods are available to equate antimicrobial activity with some characterized structures to screen broad-spectrum peptides (19).
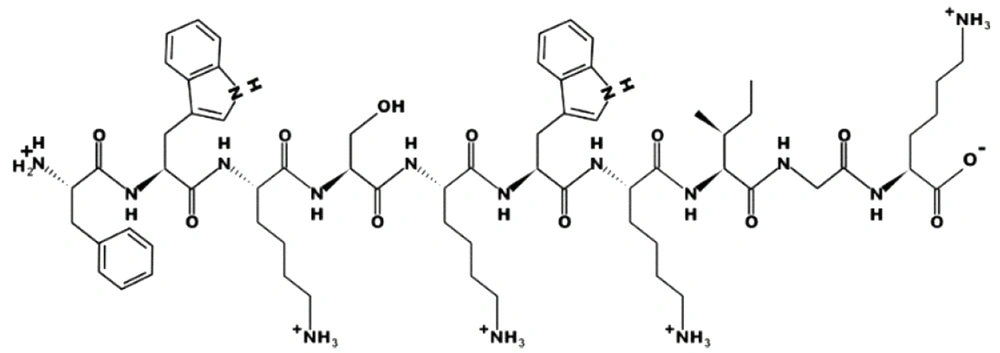
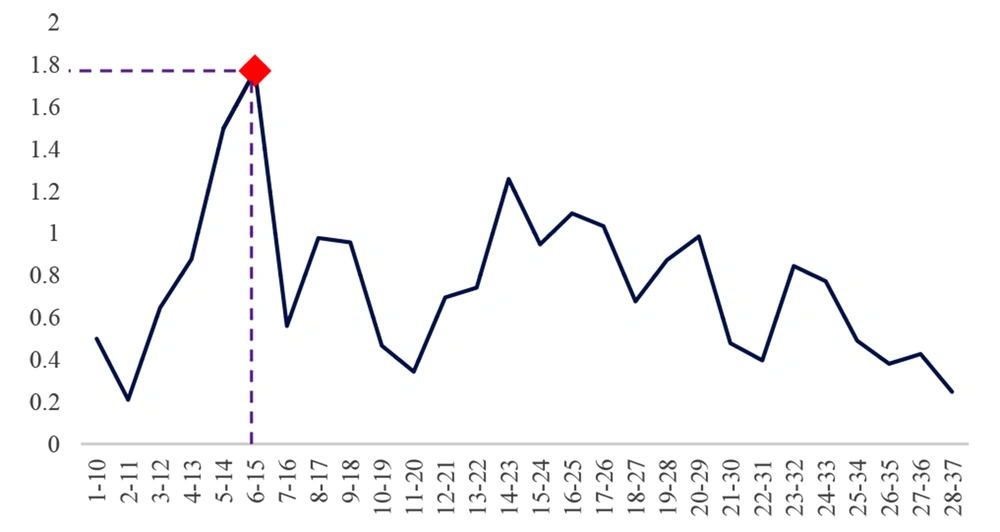
In this study, the natural human LL-37 sequence was probed in 10 amino acid frames from the 1st to 37th amino acid. The AMP probability of sequences was analyzed using bioinformatics tools to obtain the 10aa sequence with the highest score. In the next step, the selected decapeptide score by the rational design was improved and eventually led to the selection of the best decapeptide as a monomer (Catoid) (Figure 1).
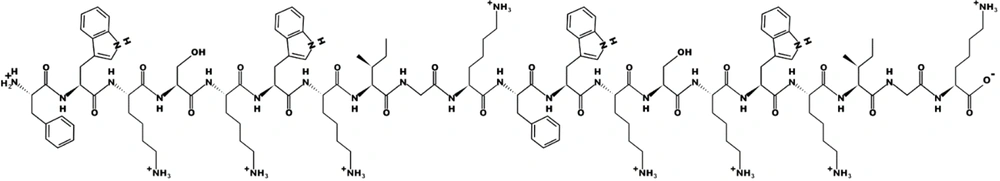
Furthermore, the dimeric analog (Figure 2) of Catoid was designed, and its activity was evaluated in silico. After the synthesis of Catoid and Dicatoid by the solid-phase method, antimicrobial activity was measured against the standard and resistant P. aeruginosa and S. aureus strains (obtained from the patient’s cornea). Then, the cytotoxic effect of AMP derivatives was determined on human fibroblast cells and bovine corneal cells.
2. Methods
2.1. Peptide Design and Bioinformatics Analysis
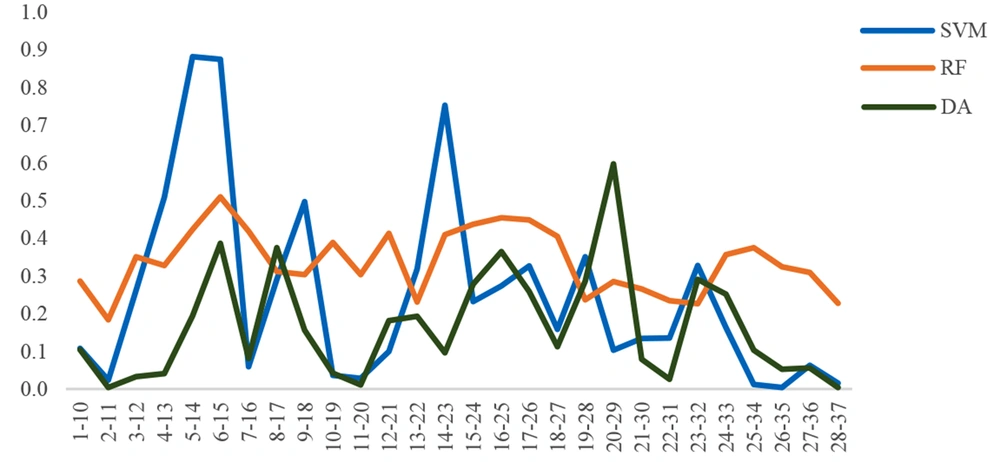
Firstly, the human LL-37 peptide sequence was obtained from the NCBI database. Amino acid frameworks from the 1st amino acid (leucine) to the 37th amino acid (serine) were selected and applied to the CAMPR3 database (http://www.camp.bicnirrh.res.in/2019) (20) to calculate AMP probability according to the results obtained from four machine learning algorithms, namely random forest, support vector machine, discriminant analysis, and artificial neural network (ANN). The threshold of each calculation is within 0.5 - 1, and the peptides are defined as an AMP if the score is higher than 0.5. According to the prediction scores and physicochemical properties, sequence 6 - 15 was selected. Some mutations were created on this sequence by the rational design to increase the AMP probability.
2.2. Synthesis of Peptides
Based on human LL-37 as a control peptide, Catoid and Dicatoid were produced by solid-phase peptide synthesis based on fluorene-9-methoxycarbonyl (Fmoc)-polypeptide dynamic ester chemistry by Mimotopes Pty Ltd (Clayton, Victoria, Australia). The peptides were purified by C18 reverse-phase high-performance liquid chromatography to 95% purity. Furthermore, to carry out peptide identification, molecular weights were determined by mass spectrometry investigations on a Sciex API100 LC/MS Mass Spectrometer (PerkinElmer Co., Norwalk, CT, USA).
2.3. Bacterial Strains, Cell line, Chemicals, and Compounds
For the evaluation of the antimicrobial activity of peptides, bacterial strains, including S. aureus ATCC 25923 and P. aeruginosa ATCC 27853 and two resistant species of the same strains (obtained from the patient’s cornea, from the Biobank of Farabi Eye Hospital of Tehran, Iran, located in the clinical laboratory of this center), were used. A human skin fibroblast cell line was used (Hu02 cell line, National Cell Bank of Iran, Pasture Institute of Iran) to conduct the cytotoxicity test.
Sabouraud dextrose agar and Sabouraud dextrose broth, Mueller Hinton Broth (MHB) and Mueller Hinton Agar, and phosphate‐buffered saline (PBS) were purchased from Gibco Company (Gibco, Carlsbad, CA, USA). Moreover, 3‐(4,5‐dimethylthiazol‐2‐yl) ‐2,5‐diphenyltetrazolium bromide (MTT) dye, glutaraldehyde, and dimethyl sulfoxide were bought from Sigma (Sigma‐Aldrich, St. Louis, MO, USA).
2.4. Evaluation of Antimicrobial Activity
For the investigation of the antimicrobial activity of the peptides, a serial dilution titration strategy was used to assign the minimum inhibitory concentration (MIC) of the peptides against four bacterial strains employed (21). Briefly, microbes were developed overnight at 37°C in MHB and diluted within the same medium. The serial dilutions of the peptides were added to the microtiter plates in a volume of 100 μL, followed by the addition of 100 μL of bacteria to give a final inoculum of 5 × 105 colony-forming units/mL.
The plates were incubated at 37°C for 24 and 48 hours, and the MIC was measured (22). Moreover, the effect of some other antibiotics, such as gentamicin, ciprofloxacin, amikacin, and penicillin, was determined on S. aureus ATCC 25923 and P. aeruginosa ATCC 27853 and two other resistant species derived from the patient’s cornea.
2.5. Toxicity of Peptides to Human Skin Fibroblast
The cytotoxicity of Catoid and Dicatoid peptides and LL-37 was evaluated by the MTT assay (23). Fibroblast Hu02 (IBRC C10309) cell line was cultured at 1 × 105 cells per well in 96-well plates in a humidified incubator at 37°C and under 5% CO2 for 24 hours. Then, the growth media with 10% FBS were removed, and the cells were rinsed two times with PBS. RPMI (Gibco, Carlsbad, CA, USA) medium with 10% FBS containing 0.5, 5, 50, 500, and 1000 µg/mL of each peptide concentration was incubated with cells for 24, 48, and 72 hours. Quintet wells were analyzed for each concentration, and column elution buffer was used as a control. Freshly prepared MTT in PBS (5 mg/mL, 10 μL) was added to each well, and the plate was incubated for an additional 4 hours. Then, the media were evacuated, and isopropanol was included at 100 µL/well. The plates were shaken delicately to encourage formazan precious stone solubilization. The absorbance was measured at 545 nm employing a microplate reader (STAT FAX 2100, USA). Half-maximal inhibitory concentration (IC50) for each peptide was calculated according to the following formula:
2.6. Cytotoxic Effect on Corneal Endothelial Cells
Fresh eyes were taken from slaughtered cows, and corneas were cut from the enucleated eyes by Dikstein and Maurice method. In this study, 0.25 g of trypan blue was dissolved in 100 mL of 0.9% saline to prepare a 0.25% solution of dye. A 0.2% solution of alizarin red was prepared by stirring 100 mL of 0.9% saline with 0.2 g of stain for 3 hours on a magnetic stirrer. Then, the solution was filtered to remove undissolved particles. In addition, a 2.48% solution of glutaraldehyde was prepared as a fixative.
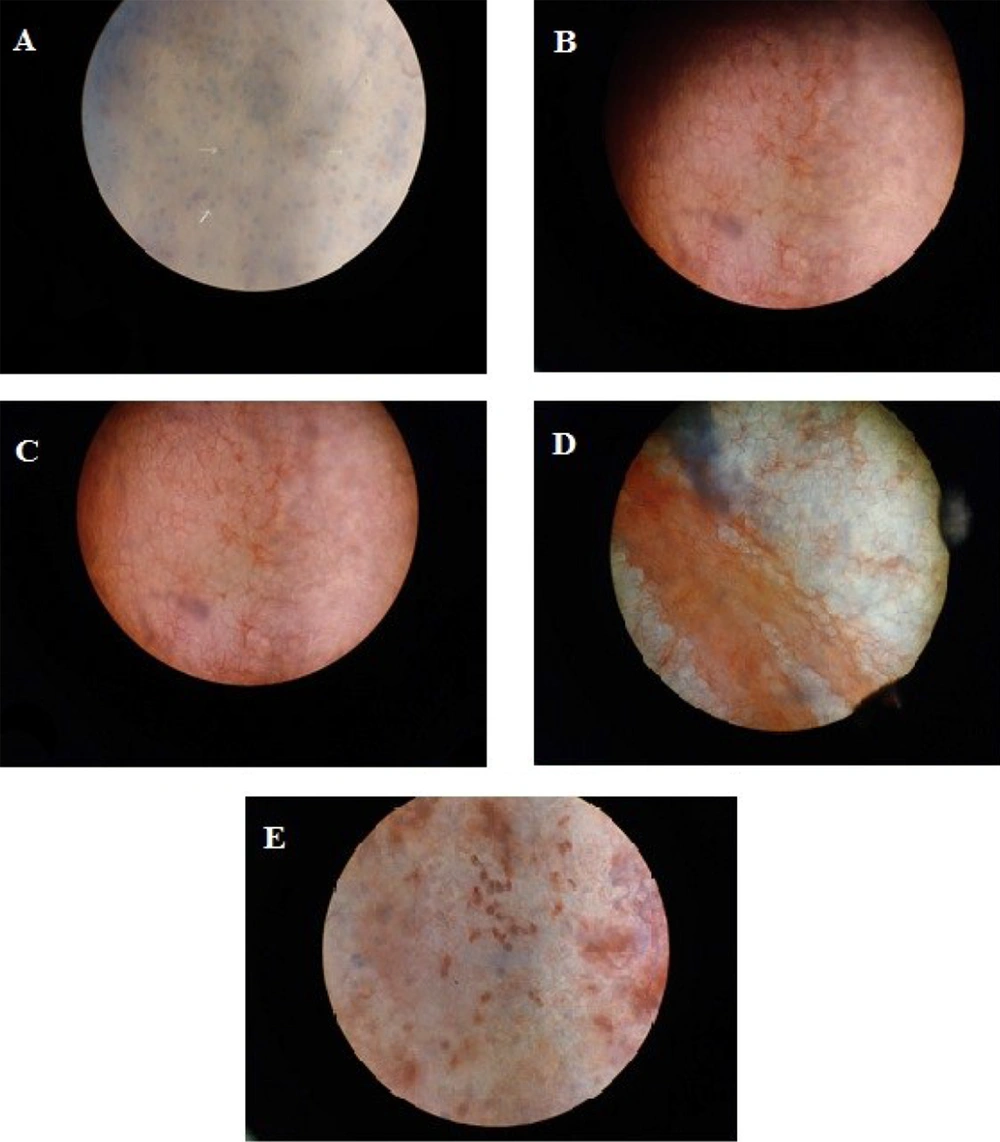
Dual staining of corneal endothelium with trypan blue (0.25%) as an indicator for damaged and permeated cells and alizarin red (0.2%) for the visualization of endothelial cell’s border was used (24). Isolated corneas (endothelial side up) were placed in a petri dish. Then, 50 µg/mL concentration of both peptides and controls (LL-37 and doxorubicin) were individually added to endothelial cells for 10 minutes. After twice rinsing with normal saline, trypan blue was added to cover the endothelium for 90 seconds. Then, the cornea was rinsed with normal saline. The same procedure was followed for alizarin red, and the cornea again was rinsed with normal saline. Following coloring, the cornea was submerged for 10 minutes in glutaraldehyde solution for fastening (Figure 3).
Finally, dual-stained and fixed corneas were placed (endothelium side up) on a microscope slide under a coverslip. Then, the damaged and undamaged cells were counted in proportion to the 200 counted cells in the four microscope fields of view.
3. Results
3.1. In Silico Peptides Design
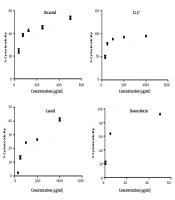
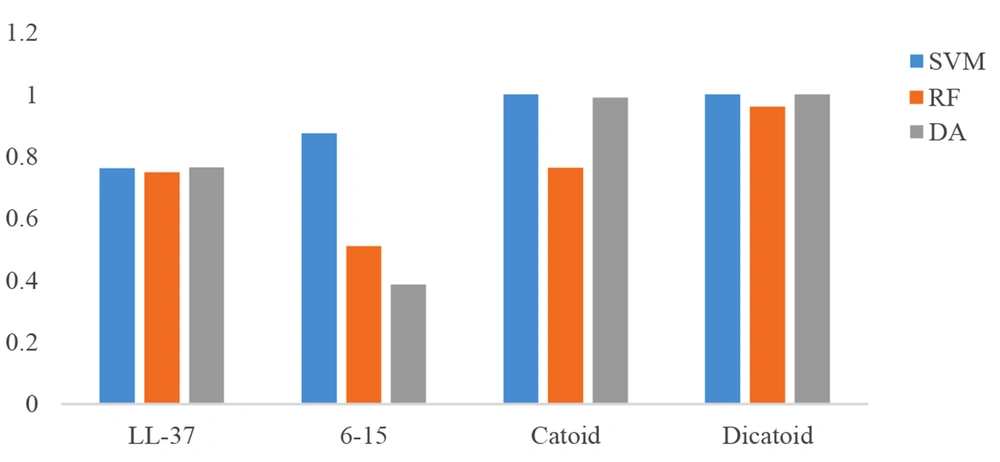
Cathelicidin peptide LL-37, with a length of 37 amino acids, was chosen to be an antimicrobial peptide that is naturally present in the eye and tears environment as a design template. The frames of 10 amino acid sequences were selected from LL-37, and the scores of those sequences were compared in the CAMP database. As reflected in Figures 4 - 5, the 6 - 15 frame was selected, which had a better result in the different algorithms. The output of the ANN algorithm is qualitative and puts the peptide chains into two categories, namely AMP and non-AMP (NAMP). This algorithm has introduced 16 of the 28 ten-amino acid peptides studied as AMP, and the 6 - 15 sequence fell into this category.
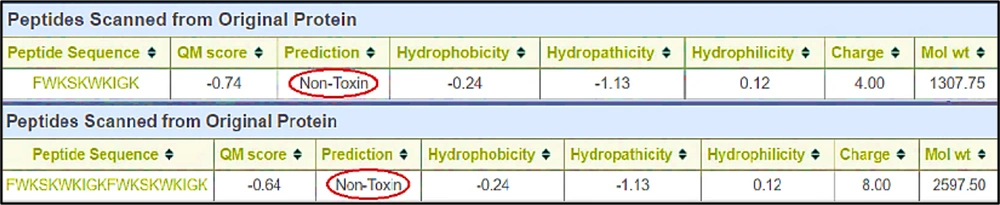
In the next step, considering the above-mentioned sequence using the rational design, the amino acids were replaced with positively charged and hydrophobic amino acids, and the antimicrobial effect was re-evaluated in silico. The primary purpose of this step was to get a score of “close to 1” in the peptide of 10 amino acids. In this regard, arginine in the second position (R2) and glutamic acid in the sixth position (E6) were replaced with tryptophan (W). Finally, FWKSKWKIGK was formed as a selected monomer (Catoid), and FWKSKWKIGK-FWKSKWKIGK was designed as a selected dimer (Dicatoid).
As shown in Figure 6, based on the results obtained from three different algorithms, the rational design of the chosen 10-amino acid peptide (6 - 15 sequence) to reach the Catoid and convert it to Dicatoid through dimerization caused a considerable growth of AMP probability score, compared to LL-37. These results promise to achieve peptides with high antimicrobial performance.
ToxinPred, an in silico method, was used to anticipate and design toxic and nontoxic peptides. As shown in Figure 7, based on the output of this software, Catoid and Dicatoid had no toxic effects on eukaryotic cells.
3.2. Minimum Inhibitory Concentration of Antibiotics and Peptides
In addition, for better results and more accurate comparisons for all four bacteria, this test was performed with LL-37, gentamicin, ciprofloxacin, amikacin, and penicillin (Table 1).
| Bacterium Name | Bacterial Strains | Antibiotic Name (µg/mL) | Peptide Name (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Gentamicin | Penicillin | Amikacin | Catoid | Dicatoid | LL37 | ||
| Standard Pseudomonas aeruginosa | ATCC 27853 | 25 | > 100 | > 100 | > 100 | 12.5 | 6.25 | 100 |
| Resistant P. aeruginosa | Clinical isolate | 50 | > 100 | > 100 | 50 | > 100 | > 100 | > 100 |
| Standard Staphylococcus aureus | ATCC 25923 | 25 | 0.78 | > 100 | 3.175 | > 100 | > 100 | 100 |
| Resistant S. aureus | Clinical isolate | 25 | 0.78 | > 100 | 12.5 | 12.5 | > 100 | 50 |
Antimicrobial Activity Evaluation of Different Agents
Among the used antibiotics, ciprofloxacin showed the highest inhibition against the standard P. aeruginosa. The designed peptides, including Catoid and Dicatoid, were significantly more effective than commercial antibiotics with a lower MIC value. In the current study, the comparison of the antimicrobial activity of Catoid and Dicatoid with LL-37 was one of the most significant endpoints. As observed, Catoid with a MIC value of 12.5 µg/ml and Dicatoid with a MIC value of 6.25 µg/mL exhibited high potency in inhibiting the growth of P. aeruginosa. Contrary to the results of the standard bacterial strain, the designed peptides did not produce good results against resistant P. aeruginosa.
Based on the results of S. aureus, Catoid and Dicatoid were not as effective as LL-37, and the best antibacterial performance in both standard and resistant samples was gentamicin. About the resistant S. aureus, high bacterial growth inhibition performance was observed in Catoid with a MIC value of 12.5 µg/mL relative to Dicatoid and even LL-37.
3.3. Evaluation of Cytotoxicity Effects on Bacteria
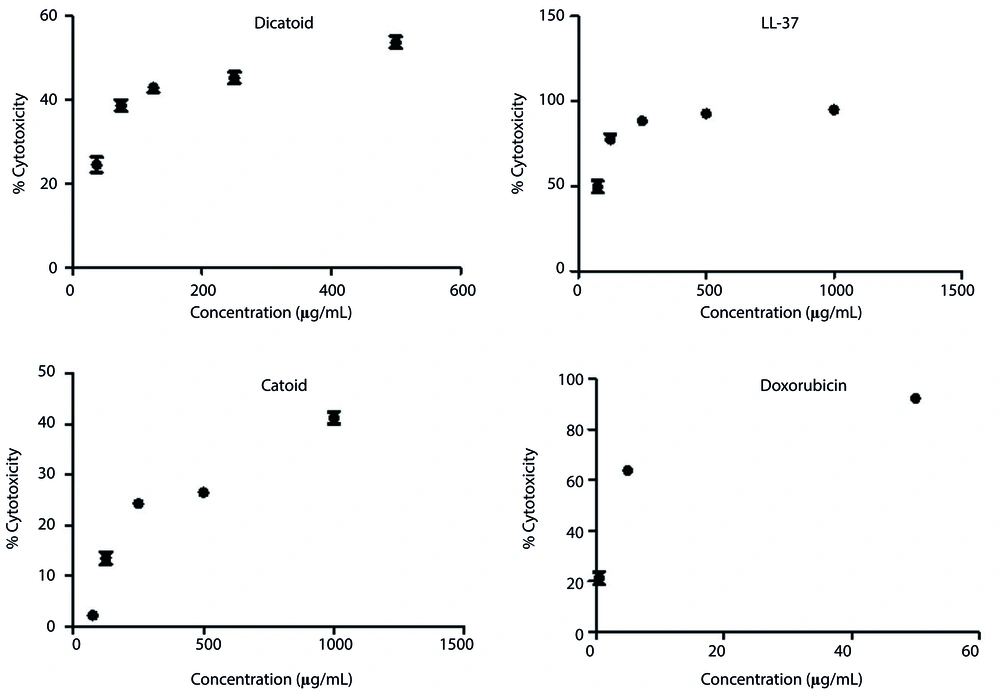
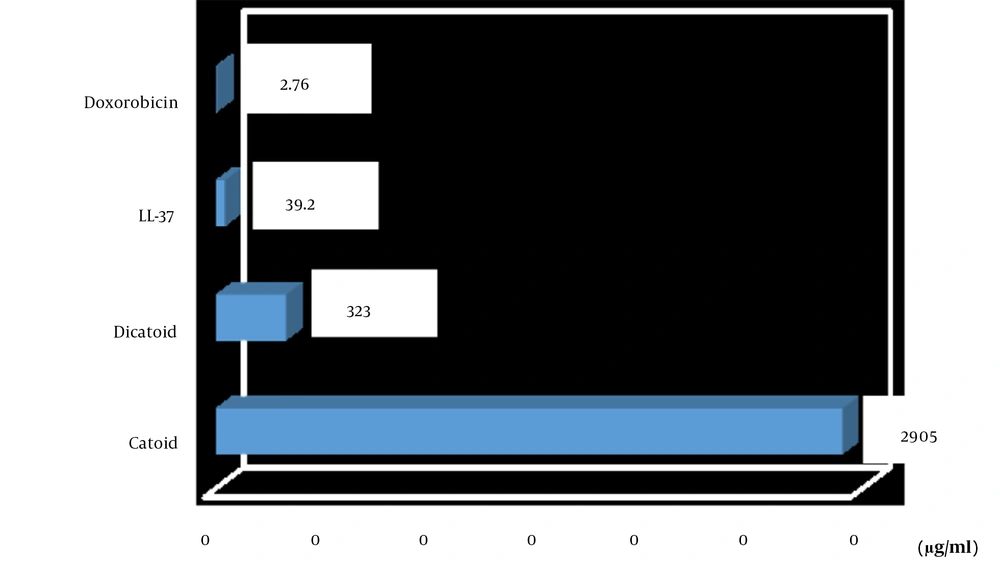
The MTT assay to determine the IC50 was carried out for Catoid, Dicatoid, and LL-37 peptides, along with doxorubicin as a positive control on human fibroblast cells (Hu02 IBRC C10309 cell line) (Figure 8).
As illustrated in Figure 9, the IC50 values of Catoid and Dicatoid were far better than LL-37. One of the surprising findings of this study was the reduction of toxicity by approximately 74 times of Catoid and 8 times of Dicatoid, compared to that of LL-37. Furthermore, a 9-fold increase in Dicatoid cytotoxicity was observed, compared to that of Catoid, which can be due to dimerization.
3.4. Evaluation of Cytotoxic Effect on Corneal Endothelial Cells
According to the scope of AMPs designed as ophthalmic antibiotics, in addition to fibroblast cells, their toxicity should be measured on corneal cells. In this regard, another method used to assess peptide toxicity is the dual staining of corneal endothelium to count the ratio of killed cells (blue) to healthy cells (red). As shown in Figure 10, Catoid and Dicatoid did not damage corneal cells at 50 µg/mL, similar to ciprofloxacin (as a negative control). However, LL-37 destroyed about 10% of the cell wall. The rate of cell destruction was estimated at about 40% when they were exposed to doxorubicin as a positive control.
4. Discussion
In silico strategy for the design of bioactive molecules are bright approaches for the reduction of the limitation of natural AMPs. The length of sequence as an important factor in natural peptides is one of the significant criteria which significantly increase the cost of preparing them by synthesis (12, 25, 26). In addition, the findings of Liu et al.’s research indicated that cytotoxic and hemolytic effects of the peptides are potentially increased by increasing the length of the chain (27). Therefore, truncation, substitution, and dimerization strategies can be helpful in tackling this problem.
The results of the present study show that, according to the forecast of antimicrobial peptide prediction databases, the position 6 - 15 in the sequence (FRKSKEKIGK) of LL-37 had the best AMP probability score, compared to other 10-amino acid frames. This finding is compatible with those previously obtained by Rice and Wereszczynski (28) and Drayton et al. (29), who reported that the presence of lysine residues for having a positive charge could improve the antibacterial activity of AMPs against both G- and G+ bacteria. Consequently, the existence of four lysine residues in this peptide (sequence FRKSKEKIGK) might lead to more AMP probability than other peptides.
Additionally, the results of the present study showed that the substitution of R2 and E6 residues with tryptophan (W) as a hydrophobic residue (sequence FWKSKWKIGK) based on rational design outputs would increase the probability of AMP activity. This outcome conforms to prior studies performed by Yin et al. (30) and Chen et al. (31), demonstrating that hydrophobicity is an important factor in the antibacterial potency of AMPs, and tryptophan as a hydrophobic amino acid can induce this effect on the current study’s 10-amino acid peptide after substitution.
According to the bioinformatic analysis, the AMP probability score of Catoid was increased after dimerization. This finding is in line with the results of a study by Gunasekera et al. (32), indicating that the dimerization of LL-37-derived peptides is an effective strategy to improve their stability and potency. However, it is noticeable that as MIC and MTT analysis demonstrated, Catoid was much more effective than the Dicatoid entity, although the IC50 of both of them was higher than LL-37. It should be noted that, despite the equality of effectiveness of Catoid and Dicatoid with LL-37, much higher doses can be used to achieve high antimicrobial effects, which is due to their extremely low toxicity, compared to that of LL-37, based on the MTT studies on fibroblast cells. Moreover, the toxicity of peptides on bovine corneal cells was inferior to that of LL-37, as the results of toxicity on the fibroblast cells were in line with the in silico predictions.
4.1. Conclusion
The present study designed and evaluated two peptides based on human LL-37 structure by in silico tools to achieve some potent novel antibiotics. In the next step, this study evaluated their antimicrobial effects against four different types of bacteria and cytotoxicity value on fibroblast cells and bovine corneal endothelium. The high antimicrobial effects of dimeric peptide and Catoid on Pseudo and Staphylococcus strains were very significant and confirmed the agreement of the theoretical and practical data. In addition, according to the toxicity prediction in ToxinPred software, the experimental data on fibroblast cells and bovine cornea showed very low toxicity of peptides, compared to that of LL-37. Other functions and effects of this new peptide, such as antibiofilm activity and mechanism of action, should be investigated in separate studies.