Introduction
Staphylococcusaureus is an opportunistic bacterial pathogen causing skin infections in hospitals, especially burn units (1). Since S.aureus can rapidly disseminated from the burn wound sites into organs via the blood stream the clinical outcome in these patients can lead to sepsis which is often fatal (2). The major problem associated with Staphylococcus infection is resistant to penicillin, methicillin or other conventional antibiotics (1). Therefore, there is the compelling need to develop novel agents and possible strategies to overcome this resistance (3). Silymarin, a flavonolignan from 'milk thistle' (Silybum marianum) plant is composed mainly of six lignans including silychristin (SC), silydianin (SD), silybin A(SBA), silybin B (SBB), isosilybin A (ISBA), and isosilybin B (ISBB) and, possesses a range of biological and medical properties, including antioxidant, anti-cancer, anti-obesity, antiviral and antibacterial activities (4-6).
Liposomes are spherical and colloidal vesicles can be used as a vehicle to drug delivery (7). These vehicles are composed of natural phospholipids, and may also contain other lipids such as cholesterol (8-11). It seems, liposome-entrapped antibiotics by increasing of bacterial membrane penetration, could reduce bacterial resistance (12, 13). Later studies demonstrated that encapsulation of plant-derived compound into liposomes markedly alters their pharmacokinetics, increasing half-lives and effectiveness (14, 15). Some of these derived such as epi-gallocatechin gallate (EGCG) and cyanidin have more antibacterial efficacy in liposomal form (12, 13). These efficacies, according to the literature, is related to type of plant-derived compound and interaction between their and liposomal membrane lipids and therefore have a major impact on therapeutic success (14, 15). The anti-MRSA effectiveness of silymarin-loaded nanoliposomes yet was not studied. The objective of this study was to prepare the silymarin-loaded nanoliposomes and evaluate its in-vitro and in-vivo antibacterial activity against isolated MRSA strain.
Experimental
Materials
Chemicals purchased from Sigma-Aldrich Chemical Company (St. Louis, USA) were silymarin, cholesterol and egg lecithin. Mueller-Hinton broth, dioxane, soybean casein digest agar (SCDA), chloroform, methanol was purchased from Merck (Darmstadt, Germany).
Microorganism
MRSA strain was isolated from clinical samples at Golestan Hospital (Ahvaz, Iran) and identified by using the reported method including tube coagulase test, slide coagulase test, latex agglutination test, Dnase and heat-stable nuclease tests, commercial biochemical tests, antimicrobial susceptibility test and PCR amplification and sequencing tests for some genes such as mecA (3, 16). This strain was inoculated onto blood agar plates and then incubated at 37 ˚C for 24 h and used for experimentation.
Preparation of nanoliposomes
Silymarin-loaded nanoliposomes were prepared using the method described previously (13). Briefly, the egg lecithin and cholesterol at the appropriate molar ratio (Table 1) were dissolved in chloroform and dried to a lipid film with a rotary evaporator (Brinkman, Toronto, Canada) under N2 flow and vacuum at 30 ˚C. The dried lipids were dispersed by agitation in silymarin solution and sonicated at 4 ˚C in ultrasonic bath (Braun-sonic 2000, Burlingame, USA). At finally, silymarin-loaded nanoliposomes were obtained by extruding of respective suspension using a polycarbonate membrane filter 100 nm-sized pores for 12 times and then for separation of excess free silymarin and larger lipid aggregation by ultracentrifugation (100000 g for 30 min). Control nanoliposomes were prepared similarly, but PBS (pH 7.4) was used instead of the silymarin solution.
| Lipids | Molar ratio of lipids (µmol/mL) |
|---|---|
| Egg lecithin:Cholestrol | 6:1 |
Determination of encapsulation efficacy
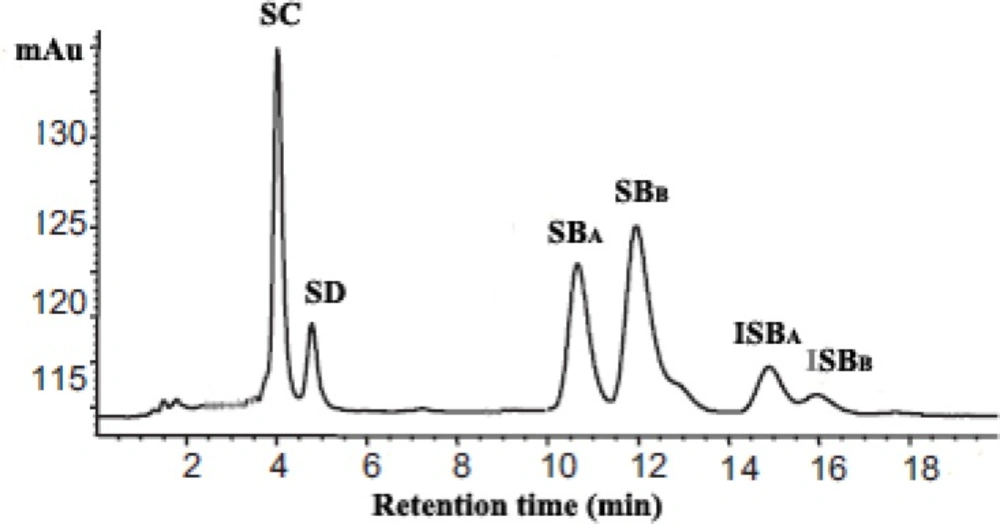
The content of silymarin in prepared nanoliposomes was determined by HPLC as previously described (17), following dissolution in 0.1% Triton X-100. To determination of silymarin, the 20 µL of nanoliposomal lysate was injected into the HPLC column. In the HPLC analysis, a C18 column (4 mm × 150 mm, 5 µm,Waters Co., Milford, USA) was used. The mobile phase was phosphoric acid (85%): methanol: water (0.5:46:64, v:v) at a flow rate of 1 mL/min. The detection was done at 288 nm. Each analysis cycle required 20 min. Silymarin corresponds to the sum of peak areas of SC, SD, SBA, SBB, ISBA and ISBA concentrations. Then, the encapsulation efficiency defined as % encapsulation = (CI/CT) × 100, where CI – silymarin in nanoliposome, CT – total silymarin in the nanoliposome preparation, was used in calculation.
Particle size, zeta-potential and polydispersity index determination
Mean particle size, polydispersity index and zeta-potential of nanoliposomes was evaluated by Malvern zetasizer (Malvern instrument, Worcestershire, UK) apparatus, as described previously (18).
Antimicrobial susceptibility testing
The MICs of free and silymarin-loaded nanoliposomes for isolated MRSA strain were determined by the broth dilution technique as recommended by Clinical and Laboratory Standards Institute (CLSI) (19). Bacterial cell suspensions (~ 5×105 cells/mL) were diluted in Mueller-Hinton broth and dispensed (100 μL) into a microtiter tray containing serial two-fold dilutions of silymarin and then incubated for 24 h at 37 ºC. The MIC was the lowest concentration of silymarin in free and nanoliposomal form that prevented visible bacterial growth and expressed in µg/mL.
Time-kill studies
Time kill studies were preformed according to the method described previously (20). Briefly, 100 µL of MRSA suspension were resuspended in 10 mL of Mueller-Hinton broth and then incubated overnight at 37 °C, and adjusted to match the 0.5 McFarland turbidity. Subsequently, 100 µL of this standardized inoculums were added to separate culture tubes containing 1 mL of Mueller-Hinton broth with 1 mL free and nanoliposomal silymarin solutions at 1, 2 and 4 times the MIC and then incubated at 37 °C. The colony counts were performed at 0, 2, 4, 6, 8, 12 and 18 h and data were expressed as log colony forming unit per milliliter (CFU/mL).
In-vivo study
In-vivo therapeutic efficacy of silymarin-loaded nanoliposomes was tested by a described method (21), with some modification. In brief, forty male BALB/c mice (20-22 g) obtained from the National Institute of Pasture, Iran. Mice were handled according with the national guidelines of the laboratory animal and housed in separate cages and received water and food ad libitum (22). Animal care and protocols were performed and approved by the Institutional Animals Ethics Committee of Borujerd Branch, Islamic Azad University (Number: 202). After anesthetized with ketamine-xylazine mixture (150 mg/Kg, given intramuscularly), the mice back’s were shaved and a brass bar (10×10×100 mm) was heated in boiling water for 18 min and then applied on the shaved back of the mice for 50 seconds to burn induction. Then, 50 μL of the bacterial inoculums (containing 1×109 CFU of total bacteria) was applied subcutaneously into the burned sites on the animal's back. The burned mice were divided into 4 groups.
Prior to the treatment starting, the gel forms of the silymarin-loaded nanoliposomes, free silymarin, empty nanoliposomes and physiological saline were prepared according to the previously described method (23).
All groups were treated topically as follows: Group 1 received silymarin-loaded nanoliposomes gel (150 mg/Kg/12h); groups 2 received free silymarin gel (150 mg/Kg/12); group 3 received empty nanoliposomes gel (150 mg/Kg/12h), and group 4 received physiological saline gel (1 mL/Kg/12h); for 12 days starting from the 4rd day post infection. Three days after the last dose the surviving mice were anesthetized and sacrificed by cervical dislocation. Then, the skin, liver and spleen of animals were removed under sterile conditions and homogenized for 5 min in PBS (pH 7.4, 2 mL/g). The homogenates were then serially diluted and plated for growth in SCDA. At finally, the inoculated plates were incubated at 35 ºC for 24 h and the colony forming unit (CFU) was counted.
Data analysis
All data were expressed as means ± SD. Statistical comparisons of killing rate study were performed by paired Student’s t-test, and p-value of less than 0.05 was considered significant. The data of survival rates were determined using ANOVA test.
Results and Discussion
Recently, the use of plant-derived compounds to eliminate of MRSA has been widely investigated (24, 25). However, the main problems associated with application of some of these components are low water solubility and low bioavailability (12, 24). To overcome of these problems, the investigators were focused on entrapment of plant-derived compounds in drug carriers such as liposomes (13). Silymarin is a mixture of flavonolignans from the medical plant Silybum marianum (17). As shown in Figure 1, the silymarin content could be estimated from sum of its six lignans peak area.
In this report, we evaluated the potential of incorporation of silymarin into nanoliposomes. Table 2 shows the zeta-potential, mean particle size, and polydispersity index of empty and silymarin-loaded nanoliposomes. Size homogeneity of empty and loaded nanoliposomes suggested that silymarin was entrapped into lipid bilayer, according to the previous studies (12, 15). Zeta-potential of nanoparticles revealed that prepared silymarin-loaded nanoliposomes have appropriate stability in aqueous dispersion (26). The results showed that silymarin can be encapsulated into nanoliposomes with high entrapment efficacy (83.00%±0.17). According to the previous studies, this phenomenon was probably due to the positive interaction between liposomal membrane lipids and loaded drugs that could be increase the encapsulation efficiacy of prepared liposomes (10, 11). It has been shown that EGCG with negative charge has high encapsulation efficacy in cationic liposomes (13). Therefore, it seems the weak forces such as the various known types of weak links between silymarin and liposomal lipids are effective factors in silymarin encapsulation.
| Formulations | Mean particle size±SD (nm) | Zeta-potential±SD (mV) | Polydispersity index±SD |
|---|---|---|---|
| Empty nanoliposomes | 93.20 ±0.11 | -1.70±0.52 | 0.31±0.07 |
The MIC values of silymarin in either free or nanoliposomal form for isolated MRSA were shown in Table 3. The MIC of silymarin-loaded nanoliposomes was lower than those of free form, respectively. Our results suggest that entrapped of silymarin in nanoliposomal form enhanced the anti-MRSA activity of its compared to free silymarin. The data from this study is according to previous report, indicating the encapsulated of oleic acid (a fatty acid found naturally in many plant) in liposomes could eliminate MRSA as well (25). Several hypotheses, including non-sensitivity of plant-derived compounds to bacterial enzymes and increased penetration of nanoliposomes into bacteria cells may explain the effectiveness of these formulations (25, 27).
| Minimum inhibitory concentration (mg/L) | ||
|---|---|---|
| Drugs | Free silymarin | Silymarin-loaded nanoliposomes |
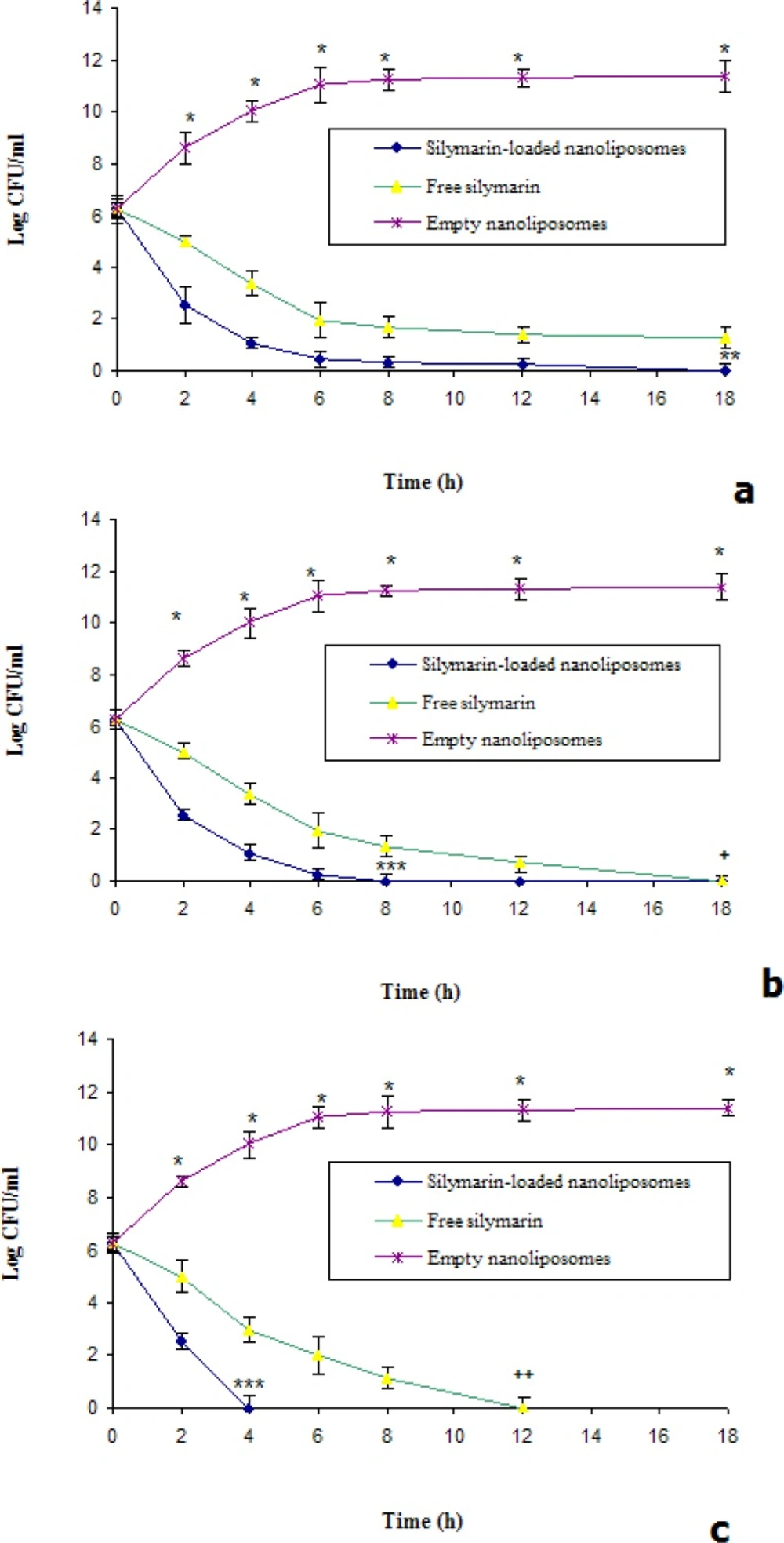
The killing curves of silymarin in free and encapsulated form at 1, 2 and 4 times the MICs were shown in Figure 2. In all conditions, silymarin-loaded nanoliposomes were more effective on reduced bacterial counts compared to free silymarin (Figure 2). At once of MIC only silymarin encapsulated in nanoliposomes could eliminate of MRSA after 18 h (Figure 2a). At twice of MIC, the encapsulated and free silymarin could eradicate the bacteria after 8 and 18 h, respectively (Figure 2b). At four times of MIC, silymarin-loaded nanoliposomes could eliminate the bacteria after 4 h (Figure 2c). Our data are accordance with previous study, which reported that significantly higher killing rates of MRSA with liposomal EGCG or oleic acid were occurred (13, 25). So, we hypothesized that interaction between the outer membrane lipopolysaccharides of S. aureus and nanoliposomes could enhance the silymarin effectiveness.
Killing curves for isolated strain S. aureus was exposed to various concentrations (a=1×MIC, b=2×MIC and c=4×MIC) of silymarin in free and nanoliposomal forms. *Significant difference between killing rate of empty nanoliposomes versus free and silymarin-loaded nanoliposomes (p<0.01), **Significant difference between killing rate of silymarin-loaded nanoliposomes versus free silymarin (p<0.05), ***Significant difference between killing rate of silymarin-loaded nanoliposomes versus free silymarin (p<0.01), +Significant difference between killing rate of free silymarin and empty nanoliposomes (p<0.05). ++Significant difference between killing rate of free silymarin and empty nanoliposomes (p<0.01).
In-vivo testing showed that the treatment of the skin infected mice with silymarin-loaded nanoliposomes could reduce significantly CFU values in evaluated organs, especially in spleen and liver (Table 4). Moreover, in other organ such as kidneys and brains of treated mice with silymarin-loaded nanoliposomes the growth of MRSA was not observed (data not shown). It was found that mortality of animals as control (without administration of silymarin) was 100% after 10 days, whereas mice treated with silymarin in free and nanoliposomal form showed the increase in survival rate of 20 and 100%, respectively. Wounds, especially burn wounds and other exposed tissues are particularly susceptible to bacterial contamination and infections (28, 29). According to previous report, the potential mortality from burn wound infections, even after aggressive antibiotics therapies, may approach 50% (30). Treatment of mice with silymarin-loaded nanoliposomes resulted in 100% survival rate and in almost complete eradication of the MRSA from the skin and spleen of infected animals. These results may be due to the optimal antibacterial delivery that reported by several investigators (31, 32). When liposomes containing antibacterial compounds are applied, they may interact with the cell membranes of bacteria and this condition can cause increased drug concentration around the bacteria (12, 25).
| Treatment | Tissue/Organ | Log CFU/Gram tissue | Percentage of survival mice (n=10) |
|---|---|---|---|
| Control without drug administration | Liver | 2.27 ± 0.40 | None survived |
| Empty nanoliposomes | Liver | 2.11 ± 0.41 | None survived |
| Free silymarin | Liver | 1.12 ± 0.43 | |
| Silymarin-loaded nanoliposomes | Liver | Nil* | 100 |
p <0.001 and
p < 0.05.
In conclusion, in-vitro and in-vivo testing of silymarin-loaded nanoliposomes indicated that this formulation has strong protective functions against MRSA and would be a good choice for treatment of patients with MRSA infections.