Introduction
Omphalocarpum procerum (Sapotaceae) is a tree confined to humid tropical Africa including Cameroon. It can grow up to 30 m in height and 40 cm in diameter. Its fruits are hard and often consumed by elephants (1, 2). In Africa, plants of the genus Omphalocarpum are prepared for various purposes such as decoctions, powders, macerations, and are used for years in traditional medicine to treat headaches; wounds skin diseases, constipation, elephantiasis, fever, cough, and rheumatism (3-6). Previous phytochemical investigations of plants of the Sapotaceae family have revealed the presence of alkaloids, phenolic compounds, glycerol, fatty acids, flavonoids, saponins and triterpenes (7-12). To the best of our knowledge, no phytochemical or pharmacological study has been reported on the species O. procerum so far. In a continuing search for bioactive compounds from Cameroonian medicinal plants, we have investigated the CH2Cl2-MeOH (1:1) extract of the pericarps of the fruits of O. procerum. Herein, we report on the isolation and structure elucidation of a new triterpenoid, procerenone (1), together with the antiparasitic activity of some isolated compounds.
Experimental
General procedure
Melting points were determined on an M-540 melting-point unit (Buchi, Flawil, Switzerland). Optical rotations were measured, in chloroform solution, on a DIP-3600 digital polarimeter (JASCO, Tokyo, Japan). Infrared (IR) spectra were determined on a Fourier transform IR spectrometer (JascoP-2000). The mass spectra were acquired on a LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a nanoelectrospray ion source. Spectra were recorded in the positive ion mode with the resolution set to 100 000. Calibration of the instrument was performed with ProteoMass LTQ/FT-Hybrid ESI positive mode calibration mix solution (Supelco Analytical, Bellefonte, PA, USA). For MS/MS experiments, the precursor ion was isolated within a window of ±1.5 m/z-units. Collision-induced dissociation (CID) was performed using Helium as the collision gas and relative collision energy of 35%. The Xcalibur software package V. 2.0.7 was used for data processing. 1H and 13C NMR spectra were recorded on a Bruker Avance II 400 MHz spectrometer equipped with a 5-mm broadband probe head (BBI), operating at 400 (1H) and 100 MHz (13C), respectively. All chemical shifts are reported as relative differences to the internal standard tetramethylsilane (TMS). Silica gels of 230- to 400-mesh and 70- to 230-mesh (Merck, Darmstadt, Germany) were used for column chromatography (CC), respectively, while aluminum sheets precoated with silica gel 60 F254 (Merck) were used for thin-layer chromatography (TLC), with various mixtures of petroleum ether, n-hexane, EtOAc, and acetone as mobile phases.
Plant material
The Shell seeds of O. procerum were collected at Ambam in the Southern province of Cameroon. The plants were identified at the National Herbarium of Cameroon, where a voucher specimen (N°11955SFR) was deposited.
Extraction and isolation
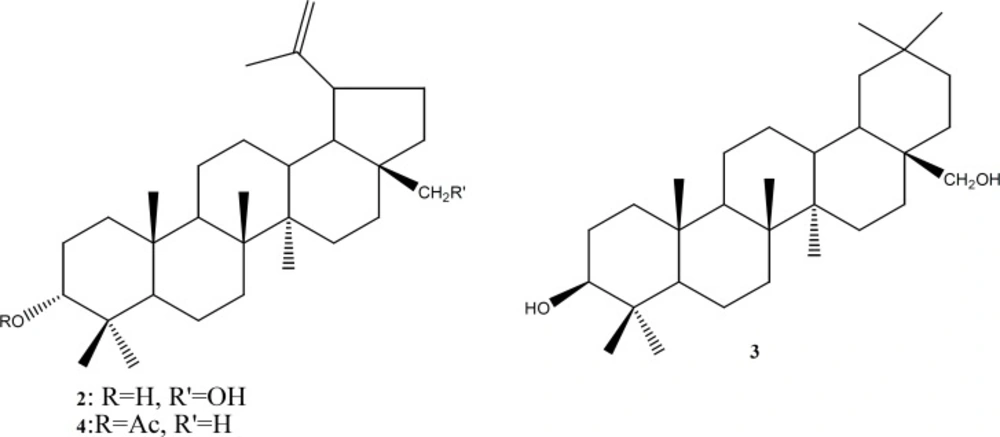
The air-dried and powdered pericarps of the fruits (1.5 Kg) of O. procerum were extracted in a CH2Cl2-MeOH (1:1) mixture (5.0 L) at room temperature within 2 days. The solvents were evaporated under reduced pressure to afford 60 g of crude extract. The resulting mixture was successively extracted with n-hexane and EtOAc at room temperature to yield 22 g and 13 g of n-hexane and EtOAc extracts, respectively. The n-hexane soluble fraction was subjected to CC over silica gel (230 – 400 mesh) and eluted with mixtures of n-hexane-EtOAc and n-hexane-CH2Cl2 of increasing polarities. A total of 121 fractions of 300 mL each were collected and combined on the basis of similar TLC profiles to yield 3 main fractions (F1-F3). Fraction F1 (8.2 g) was a complex oily mixture that was not further studied. Fraction F2 (6.4 g) was subjected to CC on silica gel (Merck, 70 – 230 mesh, Merck) and eluted with n-hexane- EtOAc (1:0 to 1:1) to afford procerenone (1) (19 mg), stigmasterol (5) (49 mg), and betulin (2) (27 mg). Fraction F3 (5.8 g) was also subjected to CC on silica gel (Merck, 70 – 230 mesh) and eluted with n-hexane-CH2Cl2 (1:4 to 0:1) to afford β-amyrin (3) (50 mg), β-sitosterol (6) (62 mg), and lupeol acetate (4) (5 mg).
Procerenone (1): 3β-hexadecanoyloxy-28-hydroxyolean-12-en-11-one
White powder. – [α]D20 41.5 (c = 0.1, CHCl3). – IR (KBr, cm-1). – νmax = 3447. 3 (OH), 1651.0 (C = O) and 1698.2 (C = O) cm-1. – 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) spectroscopic data, see Table 1. – HRESIMS: [M+H]+ at m/z = 695.5955 (calcd. m/z = 695.5973 for C46H79O4).
Bioassays
The in-vitro cytotoxicity and antiprotozoal activities against the parasites T. b. rhodesiense, T. cruzi, L. donovani, and P. falciparum were determined as earlier reported (20). The tests were carried out with the following strains, parasite forms and positive controls: T. b. rhodesiense STIB900, trypomastigote forms, melarsoprol, IC50 of 3 ng/mL; T. cruzi, Tulahuen C2C4, amastigote forms in L6 rat myoblasts, benznidazole, IC50 of 0.531 µg/mL; L donovani, MHOM/ET/67/L82, axenic amastigote forms, miltefosine, IC50 of 0.145 µg/mL; P. falciparum, NF54, erythrocytic stages, chloroquine, IC50 of 6 ng/mL and L6 cells, rat skeletal myoblasts, podophyllotoxin IC50 of 6 ng/mL.
Results and Discussion
The air-dried and ground fruit pericarp of O. procerum was extracted at room temperature with a mixture of CH2Cl2–MeOH (1:1, v/v). The extract was concentrated to dryness under vacuum and the residue subjected to repeated column chromatographic separation to yield procerenone (1) along with betulin (2) (13), β-amyrin (3) (13), lupeol acetate (4) (14), stigmasterol 5 (15), and β-sistosterol (6) (16,17).
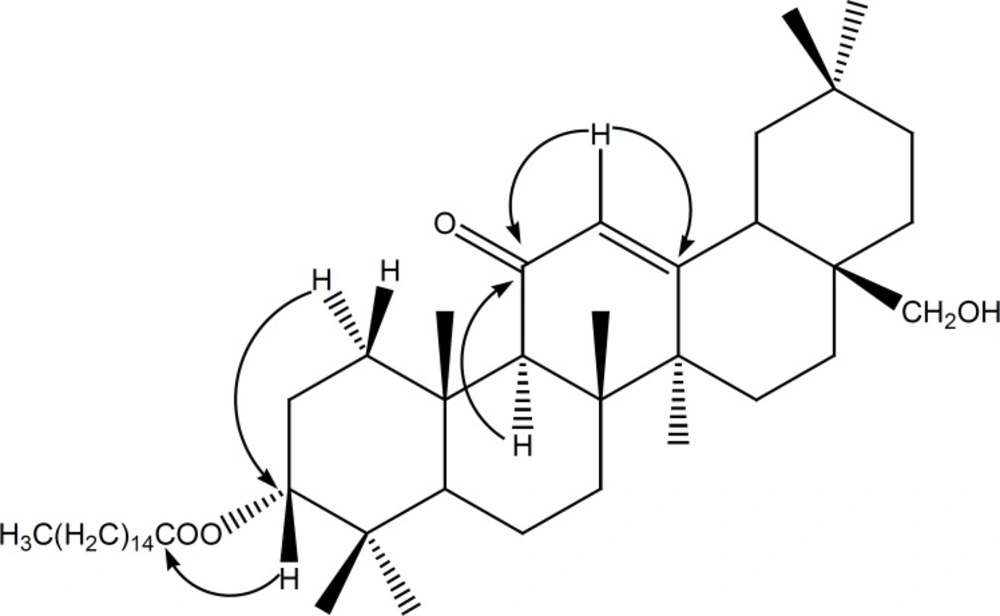
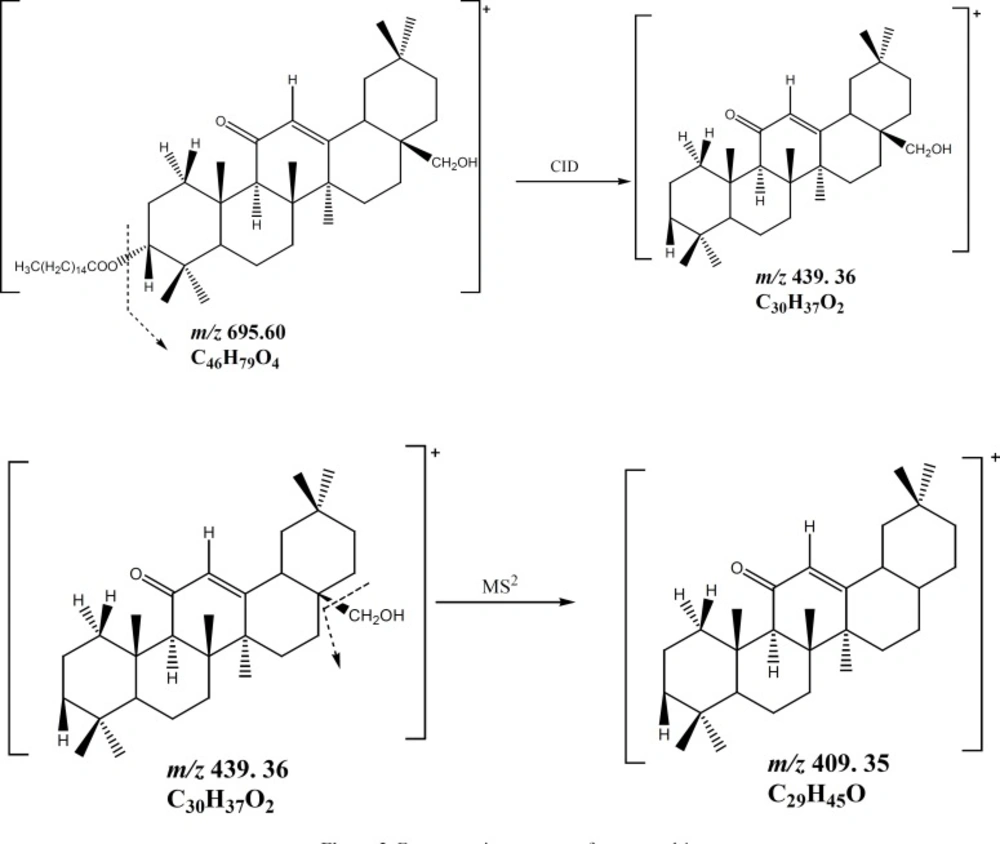
Compound (1) was obtained as a white powder. It gave a positive reaction to Liebermann-Burchard test, as usual for a triterpenoid. Its molecular formula C46H78O4, with eight double bond equivalents, was deduced from the HRESIMS spectrum which showed the pseudo-molecular ion peak [M+H]+ at m/z = 695.5955 (calcd. m/z = 695.5973 for C46H79O4). The IR spectrum showed characteristic absorption bands at 3447 (OH), 1651 and 1698 (α,β-unsaturated ketone), and 1613 (C = C) cm-1. The 1H NMR spectrum (Table 1) revealed the presence of seven singlet resonances due to seven angular triterpenoids methyl protons (δH = 0.86, 0.90, 1.10, 1.14, 1.29, 1.39 and 1.59), a singlet of one methine proton (δH = 2.37) and one singlet of an olefinic proton (δH = 5.52). This spectrum also exhibited two doublets typical of an AB system (δH = 3.45 and 3.20, J = 10.8 Hz) and one doublet of doublets (δH = 4.47, J = 4.8, 12 Hz). Furthermore, the 1H NMR spectrum exhibited series of resonances at δH = 0.88 (3H, t, J = 6.0 Hz), 1.26 (brs) and 2.27 (2H, t, J =7.5 Hz) which could be assigned to protons of a long alkyl chain. The 13C NMR (Table 1) and DEPT spectra of compound 1 displayed resonances characteristic of a single double bond (δC 128.2 and 169.6), one conjugated ketone carbonyl (δC = 199.7), one oxymethine (δC = 80.2), and one oxymethylene (δC = 69.5). The 13C NMR also confirmed the presence of the long chain acyl ester with the carbon resonances at δC = 173.5 (-COOR), 29.8 [(CH2)n] and 14.0 (CH3). The 13C NMR spectrum also displayed eight resonances typical for eight methyl groups, from which seven could be assigned to the triterpene pattern (δC = 16.3, 16.6, 18.6, 22.8, 23.2, 29.3 and 32.7) and one to a terminal methyl of the long acyl chain (δC = 14.0). On the basis of these NMR data, compound 1 was assumed to be a fatty acid ester of an olean-12-ene-type triterpenoid with one hydroxyl group and one α,β-unsaturated ketone group (12,18). The location of the ketone carbonyl at C-11 was deduced from the correlations observed in the HMBC spectrum between the olefinic proton H-12 (δH 5.52) and the carbons C-9 (δC 61.7), C-11 (δC 199.7) on one hand and between H-9 (δH 2.37) and the carbonyl carbon C-11 (δC 199.7) (Figure 1) on the other hand. According to these findings, the triterpenoid moiety of 1 was identified as being 11-oxoerythrodiol (2) (19). The ester function at the C-3 position was deduced from the correlation observed in the HMBC spectrum between H-3 (δH 4.47) and the ester carbonyl carbon (δC 173.5). The length of the acyl chain ester was deduced from the MS/MSn spectrum which exhibits the characteristic ion peaks at m/z 677.58 [M–H2O]+, m/z = 665.59 [M–CH2O]+, and m/z = 439.36 [M–C16H31O2]+ (Figure 2). The axial orientation of the H-3 proton was deduced from the coupling constants with protons H-2 (δH3= 4.47, dd, J = 4.4 and 12.0 Hz) (17). Thus, the structure of compound 1 was unambiguously assigned to 3β-hexadecanoyloxy-28-hydroxyolean-12-en-11-one, named procerenone.
Compounds 1-4 (Figure 3) were tested for cytotoxicity against L6 cell lines and for their antiprotozoal activity against Plasmodium falciparum, Leishmania donovani, Trypanosoma brucei rhodesiense and Trypanosoma cruzi. The tested compounds showed weak to moderate antiprotozoal activity against the tested parasites with IC50s in range of 9 to 80 µg/mL. No significant effect was detected regarding their cytotoxic potency.
Conclusion
The phytochemical study of the fruit pericarp of O. procerum (Sapotaceae) led to the isolation and characterization of six compounds including one new fatty acid triterpenoids, procerenone. This class of secondary metabolite has been isolated from other genera of the Sapotaceae family like Gambeya and could be considered as one chemotaxonomic marker (12). The antiprotozoal activities of the tested compounds were nearly equal to that of the extract on each tested strain of parasite. Despite the moderate antiprotozoal potency of the extract it exhibited weak cytotoxicity to L-6 cell lines. The present results partially validate the use of O. procerum in folk medicine.