1. Background
Salvia L. is one of the most important genera of Lamiaceae, known for its medicinal activity (1). The Salvia genus consists of 64 species in the Iranian flora, of which 17 are endemic (2, 3). Many studies have suggested different biological activities for sage extracts and their pure metabolites, including antiprotozoal, antifungal, antibacterial, insecticidal, and anticancer (1, 4, 5). Terpenoids and phenolics are major phytochemicals isolated from Salvia species (1). A literature survey showed a study on exudate flavonoids of S. compressa. The surface exudate of S. compressa was predominated in polar caffeic acid derivatives and contained trace amounts of apigenin and quercetin-3-methyl ether (6). Researchers have used GC and GC-MS analyses to determine the constituents of S. compressa essential oils collected from different localities of Iran, showing constituents mainly monoterpenoids, such as geraniol, nerol, borneol, and α-pinene, and sesquiterpenoids, such as β-caryophyllene, caryophyllene oxide, germacrene D, and bicyclogermacrene (7-10).
2. Objectives
Here, we report the isolation and structure elucidation of sterols and glycerides from the CH2Cl2 shoot extract of S. compressa. In addition, we determined the cytotoxic activity of the extract against MCF-7 using MTT assay and antibacterial effect against four Gram-negative bacteria and three Gram-positive bacteria using nutrient broth micro-dilution (NBMD) antibacterial bioassay.
3. Methods
1D-NMR experiments including 1H NMR (500 MHz) and APT 13C NMR (125 MHz) and 2D-NMR experiments including 1H−1H COSY, HSQC, HMBC, and NOESY were recorded on a Bruker Avance III HD 500 NMR instrument (Karlsruhe, Germany). Chromatographic techniques were applied to separate compounds, including open columns chromatography using silica gel 60 (0.063 - 0.200 mm), flash column chromatography (FCC) using silica gel 60 (0.040 - 0.063 mm), and thin layer chromatography (TLC) using pre-coated silica gel 60 F254 plates (Merck, Darmstadt, Germany). The solvents were obtained from Merck (Darmstadt, Germany). A human breast adenocarcinoma (MCF-7) cell line was obtained from the Iranian Biological Resource Center, Tehran, Iran. RPMI 1640, phosphate-buffered saline (PBS), and fetal bovine serum (FBS) were acquired from Biosera (Ringmer, UK). Penicillin/streptomycin and cisplatin were obtained from EBEWE Pharma (Unterach, Austria). Also, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was taken from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid 32% and chloramphenicol were acquired from Sigma-Aldrich. Finally, p-iodonitrotetrazolium violet (INT) was obtained from Fluka (Germany).
3.1. Plant Materials
Salvia compressa Vent. was collected in April 2017 from Fasa, Fars province, Iran (N 29° 34′; E 53° 37′; 1,444 m altitude). The plant material was identified by Mr. Mehdi Zare. Voucher specimen number PC-98-3-8-19-2 is kept at the Herbarium of medicinal and natural products chemistry research center (MNCRC), Shiraz university of medical sciences, Shiraz, Iran (2).
3.2. Extraction and Isolation
Dried shoots of S. compressa (100 g) were extracted by maceration at room temperature for 48 h with CH2Cl2 (2 × each 1 L). The crude extract (3.4 g) was vacuumed-dried at 40°C. The residue was subjected to column chromatography (50 × 5 cm) on silica gel 60. The elution was performed by applying a linear gradient from 100% n-hexane up to 100% CH2Cl2, followed by MeOH to yield 32 fractions. The similar fractions Fr.18 to Fr.21 were mixed (368 mg) and subjected to silica gel FCC using the mobile phase of diethyl ether ((C2H5)2O): n-hexane (10:90) with increasing polarity to 100 (C2H5)2O, to yield 28 fractions. Fr.18-6 was pure compound 3 (11 mg). Fr.18-21 to 18-23 were combined and subjected to preparative TLC separation using (C2H5)2O in n-hexane (50:50) to afford eight bands of which two pure compounds B18-23-1 (1, 5.5 mg) and B18-23-5 (4, 7 mg) were obtained. Fr.18 - 24 to 18 - 26 were mixed and further purified by reversed-phase semi-preparative HPLC using 100% methanol at λ of 210 nm and a 4 mL/min flow rate to yield Fr.18-25-7 as compound 2 (2.1 mg). Fr.22 to Fr.24 were pooled and loaded on 5% AgNO3-silica gel impregnated FCC (20 × 2 cm). The column was eluted using (C2H5)2O in n-hexane (50:50) with a solvent gradient to 100 (C2H5)2O, which yielded 15 fractions: Fr.22-1 to Fr.22-15. Compound 5 (0.7 mg) was purified from Fr.22-10 of the above-mentioned column.
3.3. Spectral Data
3.3.1. Compound 1
1H-NMR (500 MHz, CDCl3) δ 5.18 (dd, J = 6.4,2.0 Hz, H-7), 5.11 (q, J = 6.5Hz, H-28), 3.12 (brt, J = 10.5 Hz, H-3), 2.83 (sept, J = 7.0 Hz, H-25), 1.59 (m, overlapped, Me-29), 1.00(d, J = 6.3 Hz, Me-30), 0.98(d, J = 7.0 Hz, Me-27), 0.97 (d, J = 7.0 Hz, Me-26), 0.95 (d, J = 6.5 Hz, Me-21) 0.83 (s, Me-19), 0.54 (s, Me-18).
13C-NMR (125 MHz, CDCl3) δ 145.9 (C-24), 139.3 (C-8), 117.6 (C-7), 116.6 (C-28), 76.4 (C-3), 56.1 (C-17), 55.1(C-14), 49.8 (C-9), 46.8 (C-5), 43.5 (C-13), 40.4 (C-4), 39.7 (C-12), 37.1 (C-1), 36.7 (C-20), 36.0 (C-22), 35.0 (C-10), 31.1 (C-2), 28.7 (C-25), 28.1 (C-16), 28.1 (C-23), 26.8 (C-6), 23.1 (C-15), 21.5 (C-11), 21.3 (C-27), 21.2 (C-26), 19.1 (C-21), 15.3 (C-30), 14.3(C-19), 12.9 (C-29), 12.0 (C-18) (11).
3.3.2. Compound 2
1H-NMR (500 MHz, CDCl3) δ 5.35 (d, J = 5.1 Hz, H-6), 3.52 (dddd, J = 11.0,11.0,4.6,4.6 Hz, H-3), 1.00 (s, Me-19), 0.92 (d, J = 6.6 Hz, Me-21), 0.84 (d, J = 7.5 Hz, Me-29), 0.84(d, J = 6.8 Hz, Me-27), 0.81 (d, J = 6.8 Hz, Me-26), 0.67 (s, Me-18).
13C-NMR (125 MHz, CDCl3) δ 37.4 (C-1), 31.8 (C-2), 71.9(C-3), 42.4 (C-4), 140.9 (C-5), 121.9 (C-6), 32.0 (C-7), 32.0 (C-8), 50.2 (C-9), 36.6 (C-10), 21.2 (C-11), 39.9 (C-12), 42.4 (C-13), 56.2 (C-14), 24.4 (C-15), 28.4 (C-16), 56.9 (C-17), 12.0 (C-18), 19.5 (C-19), 36.3 (C-20), 18.9 (C-21), 34.1 (C-22), 26.1 (C-23), 45.9 (C-24), 29.1 (C-25), 20.0 (C-26), 19.2 (C-27), 23.2 (C-28), 12.1 (C-29) (12).
3.3.3. Compound 3
1H-NMR (500 MHz, CDCl3) δ 5.30 – 5.44 (m, 10H, olefinic), 5.27 (tt, J = 6.0,4.3 Hz, H-2), 4.30 (dd, J = 11.9,4.3Hz, H-1a, H-3a), 4.15 (dd, J = 11.9, 6.0 Hz, H-1b, H-3b), 2.81 (ddd, J = 7.1,7.1,5.5 Hz, 4H, double allylic), 2.77 (t, J = 6.7 Hz, 2H, double allylic), 2.31 (m, 6H, H-2′, H-2″, H-2′″), 2.06 (m, 8H, allylic), 1.98 (m, 2H), 1.62 (m, 6H, H-3′, H-3″, H-3′″), 0.97 (t, J = 7.5 Hz, methyl), 0.88 (t, J=7.0 Hz, methyl), 0.87 (t, J = 7.0 Hz, methyl) (13, 14).
13C-NMR (125 MHz, CDCl3) δ 173.4, 173.4, 172.9 (C-1′), 132.1, 130.3, 130.1, 129.8, 128.4, 128.3, 128.2, 128.0, 127.9, 127.2 (olefinic), 69.0 (C-2), 62.2 × 2 (C-1 and C-3), 34.3, 34.2, 34.1 (C-2′,2′′,2′′′), 32.0, 32.0 (C14′, C16′′), 31.6, 29.9, 29.8 × 4, 29.7 × 3, 29.6 × 2, 29.5 × 3, 29.4, 29.3 × 2, 29.2 × 4 (envelope methylene), 27.3 × 3 (allylic), 25.8, 25.7, 25.6 (double allylic), 25.0, 25.0, 24.9 (C-3′, 3′′, 3′′′), 22.8, 22.7 (C-15′, 17′′), 20.7 (C-17′′′, allylic), 14.4, 14.3, 14.2 (terminal methyls) (13, 15, 16).
3.3.4. Compound 4
1H-NMR (500 MHz, CDCl3) δ 5.43–5.30 (m, 6H, olefinic), 5.26 (quintet, J = 5.5 Hz, H-2 of triglyceride), 4.29 (dd, J = 11.8,5.5 Hz, H-1a, H-3a), 4.14 (dd, J = 11.8,5.5 Hz, H-1b, H-3b), 4.14 (m, H-2(OH) of 1,3-diglyceride), 2.81 (t, J=6.2 Hz, 2H, double allylic), 2.77 (t, J=6.7 Hz, 2H, double allylic), 2.31 (tt, J = 7.5, 4.3 Hz, 6H, H-2′, H-2′′, H-2′′′), 2.00 (m, 6H, allylic), 1.98 (m, 2H), 1.60 (m, 6H, H-3′, H-3′′, H-3′′′), 0.97(t, J = 7.5 Hz, methyl), 0.87 (t, J = 6.0 Hz, 6H, methyls) (13, 14).
13C-NMR (125 MHz, CDCl3) δ 173.4, 173.0, 172.9, 132.1, 130.4, 130.2, 129.8, 128.4, 128.4, 128.2, 128.0, 127.9, 127.2 (olefinic carbons), 69.0 (C-2), 65.2 (C-1/C-3, 1,3-diglyceride), 62.2 (C-1/C-3, triglyceride), 34.3 × 3 (C-2′, 2′′, 2′′′), 32.1, 32.0 (C14′, C16′′), 31.7, 29.9, 29.8 × 4, 29.7 × 3, 29.6, 29.5 × 3, 29.3 × 4, 29.2 × 2(envelope methylene), 27.3 × 3(allylic), 25.8, 25.8, 25.7(double allylic), 25.0, 25.0, 24.9 (C-3′, 3′′, 3′′′), 22.8, 22.8, 22.7 (C-15′, 17′′), 20.7 (C-17′′′, allylic), 14.3, 14.2, 14.2 (terminal methyls) (13, 15, 16).
3.3.5. Compound 5
1H-NMR (500 MHz, CDCl3) δ 5.42 (tq, J = 7.0,1.4Hz, H-2), 5.09 (tq, J = 7.0,1.4 Hz, H-6), 4.16 (d, J = 7.0 Hz, H-1), 2.10 (m, H-5), 2.03 (dd, J = 9.5,6.5 Hz, H-4), 1.68 (s, Me-9, Me-10), 1.61 (s, Me-8) (17).
13C-NMR (125 MHz, CDCl3) δ 140.0 (C-7), 131.9 (C-2), 124.0 (C-3), 123.5 (C-6), 59.6 (C-1), 39.7 (C-4), 26.5 (C-5), 25.8 (C-8), 17.8 (C-9), 16.4 (C-10) (18).
3.4. Cytotoxicity Assay
The cytotoxic effect of the extract was examined against cancer cells by MTT reduction assay. The MCF-7 cells were grown in complete media, consisting of RPMI1640 medium, FBS (10%v/v), antibiotics penicillin 100 units/mL, and streptomycin 100 µg/mL. The cells were cultured in humidified air containing 5% CO2 at 37°C in monolayer cultures. A 96-well test plate was used for seeding cells at a 30,000 cells/mL density, followed by incubation overnight. Afterward, the extract diluted in a growth medium was added to wells in triplicate at 3-4 different concentrations in the range of 10 to 100 µg/mL. The extract was dissolved in dimethyl sulfoxide (DMSO) and further diluted in the growth medium. The maximum DMSO concentration in the test plates was kept below 0.5%. After 72 h of incubation, the content of each well was removed, and 0.5 mg/mL MTT dissolved in RPMI medium without phenol red was added. After 4 h of incubation at 37°C, DMSO was added to solubilize the formazan crystals for an additional two hours. The absorbance of each well was measured at 570 nm, with background correction at 655 nm using a microplate reader (Bio-Rad) (5).
3.5. Antibacterial Test
The antibacterial activity of the extract was assessed against four Gram-negative bacteria, including Klebsiella pneumonia: PTCC1053, Escherichia coli: PTCC1330, Pseudomonas aeruginosa: PTCC1074, and Salmonella typhi: PTCC1609, and three Gram-positive bacteria, including Staphylococcus epidermidis: PTCC1114, Staphylococcus aureus: PTCC1112, and Bacillus subtilis: PTCC1023. The test was performed as previously described (19). Nutrient broth micro-dilution was performed in the media containing the plant extracts with final concentrations of 5 and 2.5 mg/mL, respectively. In addition, chloramphenicol was used as the positive control at the final concentrations of 0.4, 0.2, 0.1, 0.05, 0.025, and 0.0125 mg/mL in the media. For doing so, 5 μL of the extract solution or positive control in DMSO were added to 95 μL of fresh media and 100 μL of bacterial suspension in a 96-well microplate. After incubating at 37ºC for 24 h in a shaking incubator, 10 μL of 0.5% INT solution was added to the test solution and incubated for 30 minutes.
4. Results and Discussion
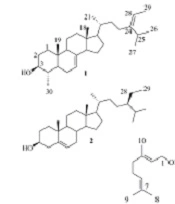
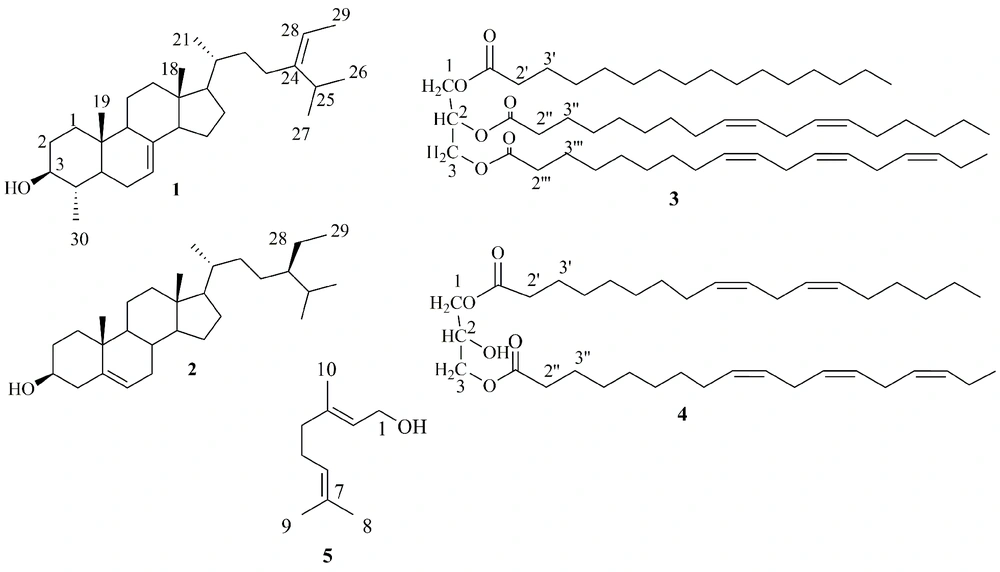
Different chromatographic techniques were used to isolate two steroids (compounds 1 and 2) and two glycerides (compounds 3 and 4), and geraniol (compound 5) from the CH2Cl2 extract of S. compressa. Spectroscopic analyses, including 1D- and 2D-NMR techniques, were employed to identify the chemical structures of the isolates. The structures of compounds 1 - 5 (Figure 1) were deduced as described below.
Compound 1 was determined as citrostadienol, (24Z) 4α-methyl-5α-stigmasta-7,24 (28)-dien-3β-ol, a phytosterol that has been isolated from different sources such as Schisandra chinensis (11) and Solanum melongena (eggplant) (20). The 1H- and 13C-NMR spectra of 1 agree well with the reported data (11, 20). The phytosterols were reported as highly anti-complementary active compounds (21). The proteins of the complement system were activated by a cascade mechanism and played an essential role in the process of allergic reactions and inflammation in addition to the host defense (11). Citrostadienol showed potent anti-complementary activity on the classical pathway with the IC50 of 4.6 × 10-8 M. Lee et al. Compared citrostadienol with other active phytosterols and concluded that it possessed the essential OH group at C-3 in addition to the C-4 methyl and C-7 double bond for the anti-complementary activity. Therefore, its high potency may be ascribed to some of these structural differences from other sterols (11).
Compound 2 was determined as β-sitosterol based on its 1H-NMR and 13C-APT-NMR spectra. The 1H-NMR spectrum showed one olefinic proton at δ 5.28, which suggested unsaturation at C-5/C-6 in the chemical structure of compound 2. The oxymethine group at δ 3.50 showed the multiplicity four times doublets with coupling constant values of 11.2, 11.2, 4.6, and 4.6 Hz, which indicate H-3α. Thus, the structure of compound 2 was identified as β-sitosterol based on the 1H- and 13C NMR spectra similarities to those presented in previous studies (12).
Compounds 3 and 4 were identified as two different glycerides. The approximate positions of the acyl substitutions on the glycerol moieties of the glycerides are deduced from the 1H- and 13C NMR chemical shifts of the respective C(H)-1 to C(H)-2 signals. The length and unsaturation degree of the acyl group does not affect the chemical shifts of the carbon signals of glycerol, but they may change the chemical shifts of the respective protons slightly (22, 23). In unsaturated fatty acyl glycerides, double bond-proton signals appear at the same range of chemical shifts (δH) that are observed for the H-2 glycerol of 2-monoglycerides, 1,2-diglycerides, and triglycerides (23). On the other hand, the 13C NMR signals of fatty acyl carbonyl of triglycerides appeared as two high- and low-frequency sets. In fact, the C = O signals located on C-1 or C-3 of the glycerol shifted by about 0.42 ppm to a higher frequency from those of carbonyls on the C-2 (22). Compounds 3 and 4 are in good agreement with these explanations.
The 13C-NMR spectrum of compound 3 showed three signals at δ 69.0 and 62.2 × 2 for C-2, C-1, and C-3 atoms, respectively. Signals at δ 172.9, 173.4, and 173.4 showed three carbonyl groups in acyl chains that confirm the position of acyl substitutions in the above-mentioned order (15). The 1H-NMR signals for the H-1 and H-3 of the glycerol moiety appeared at δ 4.30 and 4.15, respectively. The proton signal of H-2 resonated at δ 5.27, which is in the same region of olefinic protons at δ 5.30 - 5.40 (10H), suggesting the C-2 OH acylation. The presence of ten olefinic protons and corresponding carbon signals at δ 127.2, 127.9, 128.0, 128.2, 128.3, 128.4, 129.8, 130.1, 130.3, and 132.1 suggested five double bonds in the acyl chains, and their chemical shifts are similar to those reported for trilinolenin and trilinolein standard compounds (16). The signals at δ 2.77 and 2.81 represented 2H and 4H double allylic protons compatible with the presence of linoleic and linolenic acid moieties in the molecule, respectively (24). Two sets of signals at δ 2.31 and 2.06 showed three methylene H-2′, H-2′′, and H-2′′′ and eight allylic protons in acyl chains, respectively. The integrity of protons at δ 2.31 (6H) was for H-2′, H-2′′, and H-2′′′ of chains, and δ 1.62 (6H) was for protons located at H-3′, H- 3′′, and H-3′′′ of chains. The presence of the methyl signal at δ 0.97 indicated the presence of an ω3 PUFA, and two other methyl signals at δ 0.88 and δ 0.87 in acyl chains showed the presence of another unsaturated and a saturated acyl chain in compound 3 (24). Finally, the presence of 46 protons in the region δ 1.2 - 1.3 accounted for the remaining CH2 (methylene envelope) of a palmitic acid side chain together with those of the unsaturated ones, linoleic and linolenic acids.
Compound 4 was a mixture of 1,3-diglyceride and triglyceride based on the same interpretation for compound 3. The 13C-APT-NMR spectrum of compound 4 showed three signals at 69.0 (C-2), 65.2 (C-1/C-3, 1,3-diglyceride), and 62.2 (C-1/C-3, triglyceride) for the carbon atoms on the glycerol backbone. The first C-2 signal is characteristic for both 1,3-diglycerides and triglycerides, while the signal of δ 65.2 is only observed for the C-1 (3) of 1,3-diglycerides, and the last signal at δ 62.2 is considered for triglycerides (15). In addition to the presence of two proton signals of H-1b and H-3b attached to 1(3) carbon atoms of glycerol, there is another signal at δ 4.14 (3H) attributed to the presence of a free CHOH at C-2. The olefinic protons at δ 5.43 – 5.30 (6H), four double allylic signals at δ 2.81 and 2.77, and six allylic signals at δ 2.00 supported the above suggestion. The acyl groups are the same as linolenic and linoleic, as described earlier for compound 4.
Compound 5 was identified as geraniol or (2E)-3,7-dimethylocta-2,6-dien-1-ol and was isolated as a colorless oily liquid. It had a sweet, fruity, and berry-like smell, as previously described (25). Its 1H and 13C-APT-NMR spectra agreed with the reported data (17, 18). Geraniol is a valuable fragrant substance in the perfume industry and industrial synthesis of vitamins A and E (26, 27), and respells the booklouse, Liposcelis bostrychophila, and the red flour beetle (18).
The CH2Cl2 extract showed a mild effect and lowered the MCF-7 cell viability to 68.2 ± 13.1% (mean ± SEM) at the concentration of 50 µg/mL compared to the average IC50 of cisplatin, 17.0 ± 3.4 µg/mL. On the other hand, none of the bacterial strains was susceptible to the extract at 2.5 and 5 mg/mL in the NBMD method. The MIC values of the positive control, chloramphenicol, were 0.05, 0.05, 0.05, and 0.025 mg/mL for Gram-negative bacteria, including K. pneumonia, E. coli, S. typhi, and P. aeruginosa, and 0.05, 0.0125, and 0.0125 mg/mL for Gram-positive bacteria, including S. epidermidis, S. aureus, and B. subtilis, respectively. Although some Iranian Salvia species have shown good cytotoxic and antibacterial activities due to the presence of triterpenoids (28), labdane and abietane diterpenoids (4), and essential oils (19), the CH2Cl2 extract of S. compressa only showed mild cytotoxic and weak antibacterial activities.
5. Conclusions
The present research is the first report on the characterization of nonvolatile phytochemicals from the shoots of S. compressa. Although the plant extract was not an active antibacterial and showed moderate cytotoxic activity, due to the presence of the rare steroid citrostadienol, it may show potential anti-inflammatory activity in future research.