1. Background
Leishmania species, as obligate intracellular flagellate parasites, are responsible for leishmaniasis, which is an infectious disease affecting humans and animals (1). Leishmaniasis is recognized as an important vector-borne zoonotic disease, which commonly manifests as visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis, and diffuse cutaneous leishmaniasis (2). Nevertheless, limited effort has been made to control and prevent CL due to challenges, such as vector or main reservoir control. Among these challenges, the treatment of CL is the most important one (3). Different types of synthetic drugs are used for CL treatment, the majority of which have side effects and unsatisfactory therapeutic effects (4). Therefore, researchers are seeking more effective drugs to replace the current ones for the treatment of CL. Several natural compounds, including alkaloids, naphthoquinones, neolignans, chalcones, triterpenoids, and lignans, have been reported to exert inhibitory effects against Leishmania species (5).
The genus Ferula, which belongs to the Apiaceae family, is widely distributed throughout Southwest and Central Asia (e.g., Iran and Afghanistan), North India, and the Far East (6). Sesquiterpenes, coumarin, sesquiterpene lactones, monoterpene coumarins, prenylated coumarins, carbohydrates, flavonoids, phytoestrogen, and sulfur-containing derivatives are the main chemical compounds isolated from Ferula species (7). Some metabolites and extracts from some Ferula species have been identified to have pharmaceutical and biological activities, such as antifungal (8), antibacterial (9, 10), and antiviral (11) effects. Several studies have shown that Ferula species have potent antiprotozoal effects against Leishmania major (12), Leishmania tropica (13), Plasmodium falciparum (14), Trichomonas vaginalis (15), and Echinococcus granulosus (16).
In this study, considering the biological and pharmacological properties of shark cartilage extract (ShCE), such as anti-tumor, anti-angiogenesis, antioxidant, anti-inflammatory, immunomodulatory (17, 18), and leishmanicidal (19-21) effects, it was used alongside Ferula tabasensis to evaluate the synergistic or non-synergistic effects.
2. Objectives
The present study mainly aimed to evaluate the leishmanicidal activity, cytotoxicity, and apoptotic affinity of ethyl acetate extract, methanol extract, and F1-F4 fractions of F. tabasensis, used alone or in combination with ShCE against L. major in vitro.
3. Methods
3.1. Plant Samples
The roots of F. tabasensis were collected from Tehran, Iran, in July 2020. A voucher specimen (ARD-R1) was deposited in the herbarium of the School of Pharmacy of Ardabil University of Medical Sciences, Ardabil, Iran.
3.2. Isolation of Extracts and Fractions
The aerial roots of F. tabasensis (1.5 kg) were powdered and extracted with n-hexane (2×10 L), ethyl acetate (2 × 10 L), and methanol (2×10 L) by the maceration method at room temperature, respectively. The n-hexane extract was concentrated in a rotary evaporator to obtain 45 g of a dark sticky residue. The residue was then fractionated by silica gel column chromatography (230-400 mesh size, 400 g) with an n-hexane/EtOAc gradient (100:0 to 0:100) as eluent, followed by increasing concentrations of methanol (up to 20%) in ethyl acetate. Finally, F1-F4 fractions with different polarities were obtained (22). The extracts and fractions were dissolved in 1% methanol to prepare a stock solution. It is worth mentioning that previous studies have attributed no toxicity to methanol concentrations up to 1% against promastigotes or amastigotes (23).
3.3. Cultivation of L. major
The standard Iranian strain of L. major (MRHO/IR/75/ER) was prepared by Dr. Hajjaran from the School of Public Health of Tehran University of Medical Sciences (Tehran, Iran). The promastigotes were cultured and sub-cultured in the NNN and RPMI-1640 media, which were respectively enriched with 20% fetal bovine serum (FBS) inactivated at 56ºC for 30 minutes and 1% penicillin/streptomycin (Pen-Strep) at a temperature of 24°C ± 2.
3.4. J774A.1 Macrophage Cell Culture
The murine macrophage cell line, J774A.1, which was purchased from the Pasteur Institute of Iran, was cultured in RPMI-1640 medium, supplemented with 12% FBS and 1% Pen-Strep at 37°C under humidified conditions in a 5% CO2 atmosphere. The confluent cells were obtained by adding fresh media to the flasks daily (21, 24).
3.5. Preparation of ShCE
The purchased shark cartilage (Bushehr Port, Persian Gulf, Iran) was used to prepare ShCE, according to the method proposed by Hassan et al., as described in our previous studies. In brief, 10 g of cartilage powder (the cartilage was cleaned, cut into small pieces, lyophilized, and powdered) was poured into 100 mL of phosphate-buffered saline (0.1 M PBS) containing guanidine hydrochloride (4 M) and phenylmethylsulfonyl fluoride (1 mM PMSF, pH=5.8) as a protease inhibitor and incubated at 2 - 8°C for 48 hours on slight stirring. Subsequently, the solution was centrifuged at 100,000 g for 45 minutes. The supernatant was then precipitated in 20% polyethylene glycol and dialyzed against PBS. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) method was also used to evaluate the purity of proteins and represent their molecular weight against the standard protein ladder (19, 25).
3.6. Promastigote Assay
3.6.1. Parasite Counting Method
First, the microscopic parasite counting method was used to investigate the effects of various concentrations of ethyl acetate and methanol extracts and F1-F4 fractions of F. tabasensis on the promastigotes of L. major. For this purpose, 100 µL of enriched RPMI-1640 medium, containing 1 × 106 promastigotes/mL in the logarithmic growth phase, was cultured in 96-well culture plates (NuncTM) in the presence of various concentrations (1.56 - 200 µg/mL) of extracts and fractions via 24, 48, and 72 hours of incubation at 24°C. Moreover, the effects of extracts and fractions at half maximal inhibitory concentrations (IC50), combined with 200 µg/mL of ShCE, were assessed after 24, 48, and 72 hours. The final concentrations (e.g., 200 + 2.4 and 200 + 2.9 µg/mL) were prepared for combined drug formulations right before the test. Afterward, the average number of promastigotes was directly counted under a light microscope at 10× magnification. Amphotericin B (AmB, Gilead UK), prepared in sterile PBS based on the manufacturer’s instructions right before the experiments, and glucantime (GLU, 1.5 g/5 mL ampules; Sanofi-Aventis, France) were used as positive controls at different concentrations (1.56 - 200 µg/mL). The three wells of each plate without any drugs were considered as the negative controls (Ctrl-). The experiments were performed in triplicate (19, 26).
3.6.2. MTT Assay for Promastigote Viability
The 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay was used to evaluate the IC50 concentrations. For this purpose, after seeding 100 µL of adjusted RPMI-1640 medium, containing 1×106/mL logarithmic-phase promastigotes, into each of the 96-well ELISA plates, the parasites were exposed to increasing concentrations (1.56 - 200 µg/mL) of ethyl acetate and methanol extracts of F. tabasensis, as well as F1-F4 fractions. Also, 200 µg/mL of ShCE was used in combination with the IC50 concentrations of the extracts and fractions, as described earlier.
After 72 hours of incubation at 25 ± 1°C, the plates were centrifuged at 3000 rpm for 10 minutes, and the supernatant was removed and replaced with a fresh medium of the same volume. Next, 20 µL of MTT solution (5 mg MTT powder/mL in PBS) was added to each well and incubated for five hours at 37°C in a dark room. Next, the cells were centrifuged again at 3000 rpm for 10 minutes, and 100 µL of dimethylsulfoxide (DMSO) was added to the pellets. After 10 minutes, the optical densities (ODs) were measured at a wavelength of 570 nm using an ELISA reader. The viability percentage of promastigotes was determined based on the following formula:
Viability percentage = 100 × (Absorbance of treated cells - Absorbance of the blank/Absorbance of control cells - Absorbance of the blank)
Meanwhile, the promastigotes suspended in PBS with no drugs and medium with no promastigotes and drugs were respectively used as the negative control (Ctrl-) and blank (19, 27, 28).
3.6.3. Intracellular Amastigote Assay
The effects of F. tabasensis extracts and fractions on infected macrophage cells and intra-amastigotes were evaluated, as previously described (19). Briefly, 3×105 of J774A.1 macrophage (200 µL) was added to each well of 12-well plates, with small round glass coverslips at the bottom of the plate, and incubated for 12 hours at 37°C in a 5% CO2 atmosphere for adherence. Next, the suspended macrophages were removed by washing them with sterile PBS. To infect the macrophages, 3 × 106 stationary-phase promastigotes of L. major (1:10 ratio) were added to the adherent macrophages and incubated again for 12 hours at 37°C in a 5% CO2 atmosphere with 95% relative humidity.
Free parasites were removed by washing with fresh RPMI-1640 medium after 12 hours. The infected macrophages were incubated in the presence of the extracts and fractions at different concentrations (1.56 - 200 µg/mL). After 72 hours, the slides were washed with PBS, fixed with methanol, and stained with Giemsa stain to count the infected macrophages and amastigotes under an optical microscope. The GLU and AmB were used as the positive controls at the same concentrations described in the promastigote assay. The number of amastigotes in 100 macrophage cells was counted, and the IC50 was determined (19, 23).
3.6.4. Macrophage Cytotoxicity Assay
Since macrophage cells are the principal resident cells for Leishmania parasites, the J774A.1 macrophage was used in this study to assess the toxicity of ethyl acetate and methanol extracts and F1-F4 fractions of F. tabasensis at concentrations of 1.56 - 200 µg/mL, alone or in combination with 200 µg/mL of ShCE. For this purpose, 2 × 105 cells/well were cultured in 96-well microplates with RPMI-1640 medium, containing 1% Pen-Strep and 12% FBS, and the cells were allowed to attach at a temperature of 37°C in a 5% CO2 atmosphere for 12 hours. Next, 100 µL of each concentration was added to the wells and incubated for another 72 hours. Finally, cytotoxicity was determined using the MTT assay, as described in the section above. Additionally, 50% cytotoxicity concentrations (CC50) of the extracts and fractions were determined as a 50% reduction in the cell viability of treated cells relative to untreated cells. The ratio of CC50 for macrophage cells to IC50 for amastigotes was defined as the selectivity index (SI) (13, 19).
3.7. Flow Cytometry
The flow cytometry method was utilized to indicate apoptotic and necrotic cells using Annexin V Apoptosis Detection Kit (MabTag, Germany), according to the manufacturer’s instructions. In brief, 1 × 106 logarithmic-phase promastigotes, 1 × 105 uninfected macrophages, and 200 µL of infected macrophage cells, exposed to the IC50 concentrations of extracts and fractions, were collected. They were first washed with a cold, sterile PBS solution and then centrifuged for 15 minutes at 1000 g. Next, 5 µL of annexin V and propidium iodide (PI), as well as 400 µL of binding buffer, were added to the cell pellets. The promastigotes and macrophage cells were incubated at 25°C and 37°C, respectively, in a dark room for 15 minutes. The test results were read in a CyFlow Space Flow cytometer (Sysmex-Partec, USA), and the collected data were analyzed in FlowJoTM Version 10.5.3 (Vancouver, BC, Canada). All the experiments were performed in triplicate, and the percentages of apoptosis and necrosis were assessed for each tested sample.
3.8. Statistical Analysis
Data are presented as mean and standard deviation (SD) of experiments run in triplicate. The IC50 values of promastigotes and amastigotes, as well as the CC50 values of J774A.1 macrophage cell were calculated based on the mean viability percentage of promastigotes, amastigotes, and macrophage cells, respectively, against the untreated controls. Microsoft Excel Version 16 and SPSS Version 24 (SPSS Inc., Chicago, IL, USA) was used for evaluating the results, drawing figures, and analyzing the data. Moreover, the repeated measures ANOVA test was used to analyze the results of the parasite counting method. A one-way ANOVA test, followed by Tukey's post-hoc test, was also performed to compare the IC50 and CC50 values of compounds. Finally, the SI value was calculated by determining the ratio of CC50 to IC50 for amastigotes.
4. Results
4.1. Parasite Counting Method
According to the microscopic analysis, the antileishmanial activity of F. tabasensis extracts and fractions against L. major promastigotes varied in a dose- and time-dependent manner. The number of promastigotes was markedly reduced by increasing the concentrations of extracts and fractions and also over time (from 24 to 72 hours) compared to the negative control (CTRL-) (P < 0.05). The F1 and methanol extracts were more effective against promastigotes compared to the ethyl acetate extract and other fractions (Table 1). Overall, the present results highlighted the remarkable effects of ShCE at a concentration of 200 µg/mL in combination with the IC50 concentrations of F. tabasensis extracts and fractions against the promastigotes (P < 0.05) (Table 2).
| Variables | Concentration (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 100 | 50 | 25 | 12.5 | 6.26 | 3.12 | 1.56 | Control - | |
| Ethyl acetate | |||||||||
| 24 | 23.2 ± 2.8 | 18.3 ± 1.5 | 17.1 ± 1.7 | 26.7 ± 5.4 | 35.3 ± 3.2 | 30.2 ± 2.8 | 46.5 ± 4.5 | 50 ± 7.3 | 91.3 ± 1.5 |
| 48 | 21.7 ± 3.5 | 16 ± 4.1 | 13.5 ± 2.7 | 23 ± 2.1 | 31 ± 3.9 | 29.3 ± 3.5 | 42.6 ± 2.9 | 45.4 ± 3.2 | 97.5 ± 2.6 |
| 72 | 17.2 ± 5.3 | 14 ± 3.6 | 11.3 ± 5.4 | 18.7 ± 4.5 | 25.3 ± 3.9 | 27 ± 3.7 | 40 ± 2.5 | 42 ± 6.7 | 102.6 ± 38 |
| Methanol | |||||||||
| 24 | 3.6 ± 3.1 | 7.3 ± 5.1 | 10.6 ± 2.3 | 11.3 ± 5.1 | 24.3 ± 1.1 | 28 ± 2.1 | 31.6 ± 6.1 | 33 ± 7.2 | |
| 48 | 2.3 ± 4.5 | 2.3 ± 6.6 | 8.3 ± 7.1 | 9 ± 1.2 | 17.3 ± 3 | 24.6 ± 2.5 | 29 ± 6.2 | 30.6 ± 4.5 | |
| 72 | 0 ± 1.1 | 1.6 ± 0.8 | 3.4 ± 1.2 | 5.1 ± 3.2 | 14.2 ± 3.5 | 22.6 ± 1.5 | 26.5 ± 2.9 | 29 ± 2.1 | |
| F1 | |||||||||
| 24 | 3 ± 1.1 | 3 ± 1.5 | 9.4 ± 2.3 | 12.3 ± 6.1 | 22.7 ± 1.4 | 40.3 ± 1.9 | 51.7 ± 3.1 | 65.3 ± 6.3 | |
| 48 | 1.7 ± 1.6 | 2 ± 2.1 | 7 ± 4.5 | 10 ± 5.1 | 22 ± 2.1 | 36 ± 1. 7 | 51.6 ± 3.1 | 61.5 ± 5.9 | |
| 72 | 0 ± 0.85 | 0.5 ± 1.9 | 3.3 ± 2.4 | 9.5 ± 1.6 | 19 ± 4.1 | 32.7 ± 2.7 | 49.6 ± 3.1 | 60.7 ± 5.1 | |
| F2 | |||||||||
| 24 | 3.3 ± 1.4 | 5.2 ± 1.9 | 10.3 ± 3.1 | 24.7 ± 1.8 | 35.3 ± 5.2 | 54.3 ± 3.5 | 64 ± 1.6 | 74 ± 3.3 | |
| 48 | 2 ± 1.1 | 5 ± 1.1 | 9.1 ± 1.13 | 20.3 ± 1.1 | 34.7 ± 1.1 | 45.2 ± 1.1 | 58 ± 1.1 | 72.3 ± 1.1 | |
| 72 | 0.2.1 | 4 ± 4.1 | 8.6 ± 1. 7 | 18 ± 3.2 | 31.3 ± 3.3 | 42.7 ± 3.4 | 56 ± 0.9 | 70.6 ± 1. 7 | |
| F3 | |||||||||
| 24 | 2 ± 1.8 | 5.3 ± 1.3 | 12.2 ± 1.7 | 35.5 ± 4.1 | 49 ± 2.1 | 64.3 ± 2.2 | 72.3 ± 1.6 | 75.7 ± 1.3 | |
| 48 | 1.3 ± 3.3 | 4 ± 2.1 | 11.3 ± 4.3 | 31.2 ± 1.7 | 45 ± 5.1 | 60 ± 4.5 | 69 ± 1. 3 | 73.8 ± 6.1 | |
| 72 | 0 ± 1.7 | 3.2 ± 1.3 | 10 ± 6.1 | 30.4 ± 2. 3 | 42.7 ± 7.1 | 58.4 ± 2.1 | 61.4 ± 3.1 | 67.3 ± 5.1 | |
| F4 | |||||||||
| 24 | 17 ± 1.4 | 29 ± 3.7 | 36.3 ± 1.3 | 45 ± 3.1 | 52.6 ± 1. 7 | 60.6 ± 1.4 | 68 ± 2.5 | 75.3 ± 4.3 | |
| 48 | 16.2 ± 4.6 | 27 ± 1.9 | 33.3 ± 1.2 | 41.4 ± 4.1 | 45 ± 5.1 | 54.2 ± 2.5 | 61.1 ± 3.1 | 72.1 ± 4.5 | |
| 72 | 15 ± 1.7 | 25 ± 2.6 | 31 ± 3.1 | 38 ± 1.3 | 40.5 ± 1.9 | 48 ± 3.4 | 56 ± 2.2 | 71.8 ± 7.1 | |
| AmB | |||||||||
| 24 | 14 ± 3.1 | 21 ± 2.5 | 27 ± 3.6 | 37 ± 2.1 | 55 ± 1.8 | 58 ± 2.4 | 62 ± .5 | 65 ± 0.8 | |
| 48 | 10 ± 1.1 | 16 ± 1.6 | 21 ± 2.5 | 29 ± 2.2 | 51 ± 2.7 | 55 ± 1.9 | 57 ± 1.2 | 60 ± 1.1 | |
| 72 | 5 ± 1.3 | 11 ± 4.1 | 17 ± 1.8 | 22 ± 2.5 | 45 ± 4.1 | 50 ± 2.8 | 52 ± 3.5 | 57.3 ± 2.1 | |
| GLU | |||||||||
| 24 | 83 ± 1.5 | 84 ± 1.5 | 85 ± 1.1 | 86 ± 1.2 | 87 ± 1.1 | 88 ± 2.5 | 90 ± .2 | 91 ± 1.8 | |
| 48 | 75 ± 1.5 | 80 ± 1.8 | 84 ± 2.1 | 87 ± 2.5 | 92 ± 2.1 | 94 ± 1.2 | 96 ± 1.5 | 97 ± 1.5 | |
| 72 | 50 ± 1.9 | 55 ± 2.1 | 57 ± 1.5 | 59 ± 2.3 | 62 ± 1.1 | 67 ± 2.5 | 69 ± 1.5 | 71 ± 2.9 | |
a Data are expressed as mean ± SD.
| Variables | Concentration (µg/mL) | |
|---|---|---|
| ShCE (200 µg/mL) | Control - | |
| ShCE (200 µg/mL) | ||
| 24 | 48 ± 2.5 | 91.3 ± 1.5 |
| 48 | 42 ± 2.3 | 97.5 ± 2.6 |
| 72 | 35 ± 1.8 | 102.6 ± 3.8 |
| Ethyl acetate (3.8 µg/mL) | ||
| 24 | 27.8 ± 2.3 | |
| 48 | 24.5 ± 1.5 | |
| 72 | 11.2 ± 2.1 | |
| Methanol (2.9 µg/mL) | ||
| 24 | 17 ± 3.3 | |
| 48 | 12 ± 1.5 | |
| 72 | 9 ± 2.6 | |
| F1 (2.4 µg/mL) | ||
| 24 | 21 ± 2.6 | |
| 48 | 16 ± 2.3 | |
| 72 | 12 ± 1.5 | |
| F2 (4.85 µg/mL) | ||
| 24 | 37.5 ± 3.2 | |
| 48 | 30 ± 1.3 | |
| 72 | 22.8 ± 1.9 | |
| F3 (4.2785 µg/mL) | ||
| 24 | 33.8 ± 3.8 | |
| 48 | 25.5 ± 2.3 | |
| 72 | 16.7 ± 1.2 | |
| F4 (33.5 µg/mL) | ||
| 24 | 42 ± 1.6 | |
| 48 | 36.9 ± 2.2 | |
| 72 | 32 ± 1.9 | |
| AmB (33.9 µg/mL) | ||
| 24 | 23 ± 2.1 | |
| 48 | 17 ± 1.5 | |
| 72 | 12 ± 3.7 | |
| GLU (420 µg/mL) | ||
| 24 | 73 ± 1.9 | |
| 48 | 65 ± 1.3 | |
| 72 | 43 ± 1.4 | |
a Data are expressed as mean ± SD.
4.2. MTT Assay for Promastigote Viability
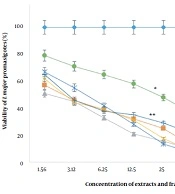
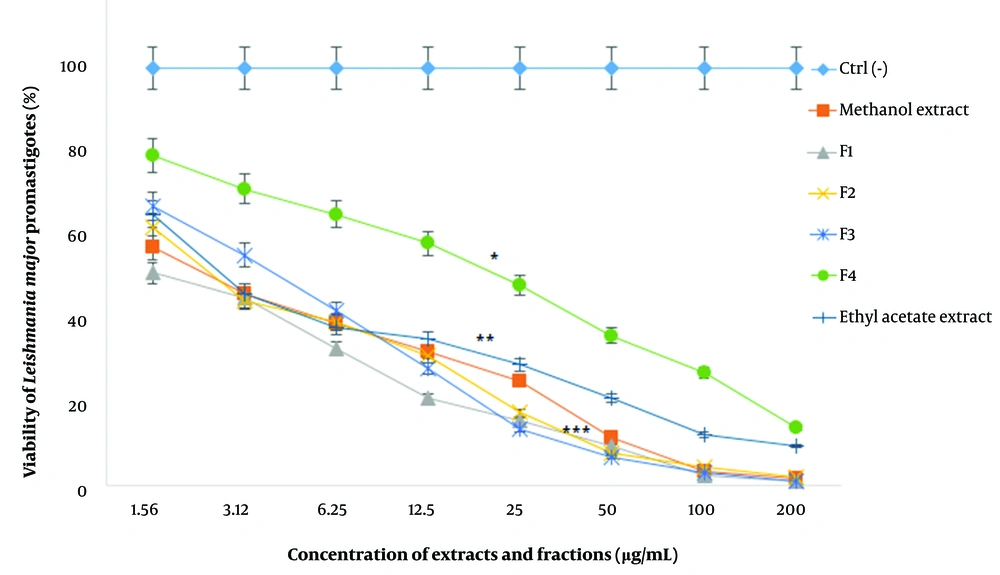
Based on the results, the viability of promastigotes against various concentrations of extracts and fractions (1.56 - 200 µg/mL) decreased in a dose-dependent manner and efficacy increased with an enhancement of drug concentrations. In other words, concentrations of 1.56 and 200 µg/mL resulted in the highest and lowest viability percent, respectively (P < 0.001).
The IC50 values of ethyl acetate extract, methanol extract, and F1-F4 fractions were 3.8 ± 1.1, 2.9 ± 0.5, 2.4 ± 0.2, 4.8 ± 1.2, and 4.2 ± 1.8, and 33.5 ± 2.6 µg/mL, respectively (Figure 1). The highest and lowest IC50 values were attributed to the F4 and F1 fractions, respectively (P < 0.001). Also, the IC50 values of AmB and GLU, as the positive controls, were measured to be 33.9 ± 5.1 and 420 ± 1.9 µg/mL for the promastigotes, which were significantly higher than the other extracts and fractions (P < 0.001).
The viability percentage of L. major promastigotes against different concentrations (µg/mL) of F. tabasensis extracts and fractions after 72 hours. Data are shown as mean ± SD of triplicate experiments. The results are analyzed by Dunnett’s test (*P < 0.05, **P < 0.01, *** P < 0.001, compared to the control group (Ctrl-)).
The results of statistical analysis indicated significant differences between all the groups in comparison to the control group (P < 0.05).
The results of statistical analysis indicated significant differences between all the groups compared to the control group (P < 0.05).
4.3. Amastigote Assay
The results of the amastigote assay revealed that different concentrations of F. tabasensis extracts and fractions resulted in a remarkable reduction in both the number of infected macrophages and the number of intra-amastigotes in comparison to the negative control (Ctrl-) (P < 0.05). Overall, 42.5% of J774A.1 macrophage cell was infected in the Ctrl- group. Concerning the effects of exposure to the extracts and fractions, it was found that the ethyl acetate and methanol extracts, F1 fraction, and F3 fraction significantly decreased the number of infected macrophage cells, as well as the mean number of intracellular amastigotes at different concentrations compared to the other studied compounds and positive controls (P < 0.001) (Table 3). Interestingly, the IC50 was lower than the IC50 calculated for the promastigotes, except for the F2 fraction (Table 4). On the other hand, ShCE at 200 µg/mL, combined with various concentrations of extracts or fractions, significantly reduced the number of infected macrophages, as well as the mean number of intra-amastigotes (P < 0.001); therefore, its combined use with F. tabasensis extracts and fractions was more efficient than its independent use (data are not shown).
| Variables | Concentration (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 100 | 50 | 25 | 12.5 | 6.26 | 3.12 | 1.56 | Control - | |
| Ethyl acetate | |||||||||
| Infected Mϕ (%) | 15 ± 1.4 | 19 ± 2.8 | 24.8 ± 3.2 | 33 ± 2.1 | 37.5 ± 2.4 | 38 ± 2.7 | 39 ± 3.1 | 40.4 ± 1.3 | 42.5 ± 2.1 |
| Amastigotes/macrophages | 0.08 ± 0.01 | 0.11 ± 0.03 | 0.29 ± 0.07 | 0.62 ± 0.06 | 1.1 ± 0.05 | 1.98 ± 0.1 | 2.29 ± 0.5 | 3.29 ± 0.5 | 5.33 ± 0.33 |
| Methanol | |||||||||
| Infected Mϕ (%) | 13 ± 2.4 | 17 ± 1.9 | 25. ± 1.1 | 31 ± 0.9 | 35 ± 1.1 | 39 ± 2.5 | 40 ± 0.4 | 40 ± 1.2 | |
| Amastigotes/macrophage | 0.02 ± 0.03 | 0.05 ± 0.03 | 0.13 ± 0.03 | 0.48 ± 0.05 | 0.96 ± 0.06 | 1.66 ± 0.05 | 2.11 ± 0.08 | 2.35 ± 0.1 | |
| F1 | |||||||||
| Infected Mϕ (%) | 10 ± 2.5 | 15 ± 1.2 | 25 ± 1.6 | 30 ± 0.5 | 35 ± 1.1 | 39 ± 2.9 | 40 ± 0.4 | 40 ± 1.7 | |
| Amastigotes/macrophages | 0.05 ± 0.03 | 0.09 ± 0.01 | 0.21 ± 0.96 | 0.85 ± 0.05 | 1.12 ± 0.07 | 1..85 ± 0.09 | 2.1 ± 0.05 | 2.85 ± 0.08 | |
| F2 | |||||||||
| Infected Mϕ (%) | 21 ± 2.1 | 25 ± 0.2 | 31 ± 3.6 | 35 ± 2.5 | 39 ± 1.1 | 39 ± 1.6 | 39 ± 1.7 | 40 ± 1.3 | |
| Amastigotes/macrophages | 0.92 ± 0.12 | 0.96 ± 0.07 | 1.15 ± 0.17 | 1.7 ± 0.05 | 2.1 ± 0.15 | 2.38 ± 0.11 | 3.2 ± 0.21 | 4.5 ± 0.1 | |
| F3 | |||||||||
| Infected Mϕ (%) | 17 ± 2.1 | 21 ± 1.2 | 25 ± 3.1 | 33 ± 3.5 | 35 ± 1.8 | 38 ± 2.6 | 39 ± 1.4 | 40 ± 1.3 | |
| Amastigotes/ macrophage | 0.23 ± 0.05 | 0.85 ± 0.07 | 0.96 ± 0.01 | 1 ± 0.03 | 1.1 ± 0.09 | 1.5 ± 0.23 | 1.89 ± 0.05 | 2.95 ± 0.1 | |
| F4 | |||||||||
| Infected Mϕ (%) | 26 ± 1.1 | 28 ± 2.7 | 31 ± 3.1 | 36 ± 2.8 | 37 ± 4.2 | 39 ± 0.7 | 40 ± 0.2 | 40 ± 1.1 | |
| Amastigotes/macrophages | 0.99 | 1.1 | 1.55 | 2.79 | 3.26 | 3.95 | 4.26 | 5.25 | |
| AmB | |||||||||
| Infected Mϕ (%) | 28 ± 0.2 | 31 ± 1.1 | 34 ± 0.8 | 36 ± 0.5 | 39 ± 0.2 | 39 ± 1.6 | 40 ± 1.2 | 40 ± 1.3 | |
| Amastigotes/macrophages | 1.1 ± 0.21 | 1.85 ± 0.15 | 2.58 ± 0.31 | 3.2 ± 0.29 | 3.8 ± 0.52 | 4 ± 0.18 | 4.3 ± 0.21 | 4.9 ± 0.11 | |
| GLU | |||||||||
| Infected Mϕ (%) | 25 ± 1.5 | 31 ± 2.5 | 34 ± 1.5 | 35 ± 1.2 | 38 ± 1.5 | 41 ± 0.5 | 42 ± 0.5 | 42 ± 1.3 | |
| Amastigotes/macrophages | 2.75 ± 0.14 | 3.1 ± 0.08 | 3.9 ± 0.23 | 4.5 ± 0.11 | 4.7 ± 0.09 | 4.9 ± 0.1 | 5 ± 0.02 | 5.1 ± 0.11 | |
Abbreviation: Mφ, macrophages.
a Data are expressed as mean ± SD.
| Extracts and Fractions | Promastigotes, IC50 ± SD (µg/mL) | Amastigotes, IC50 ± SD (µg/mL) | J774 Macrophage cells, CC50 ± SD (µg/mL) | SI=CC50/IC50 of Amastigotes |
|---|---|---|---|---|
| Ethyl acetate | 3.8 ± 1.13 | 2.95 ± 0.26 | 159.9 ± 4.2 | 53.9 |
| Methanol | 2.9 ± 0.55 | 1.39 ± 0.68 | 76 ± 7.9 | 54.7 |
| F1 | 2.4 ± 0.29 | 1.79 ± 0.27 | 123.9 ± 1.8 | 69.2 |
| F2 | 4.85 ± 1.2 | 5.92 ± 0.08 | 19.5 ± 2.6 | 1.6 |
| F3 | 4.27 ± 1.82 | 2.25 ± 0.58 | 132.8 ± 3.1 | 59 |
| F4 | 33.5 ± 2.66 | 28.9 ± 0.25 | 295 ± 15.2 | 10.2 |
| GLU | 420 ± 1.9 | 210 ± 2.3 | 845 ± 10.5 | 4.02 |
| AmB | 33.9 ± 5.1 | 55.6 ± 0.21 | 315 ± 9.8 | 5.7 |
Abbreviations: IC50, 50% inhibitory concentration; CC50, 50% cytotoxicity concentration, SI: ratio of toxicity toward macrophage cells to toxicity toward amastigote cells.
The results of statistical analysis revealed significant differences between all groups compared to the control group (P < 0.05).
4.4. Cytotoxicity of Macrophages
Similar to promastigotes and amastigotes, the cytotoxicity of macrophages was based on the concentrations of extracts and fractions (P < 0.05). Evaluation of all extracts and fractions, except F2, indicated the low toxic effects of macrophages (SI>10). As shown in Table 4, the highest and lowest IC50 values were 295 ± 15.2 and 19.5 ± 2.6 µg/mL, attributed to the F4 and F2 fractions, respectively (Table 4).
4.5. Flow Cytometry
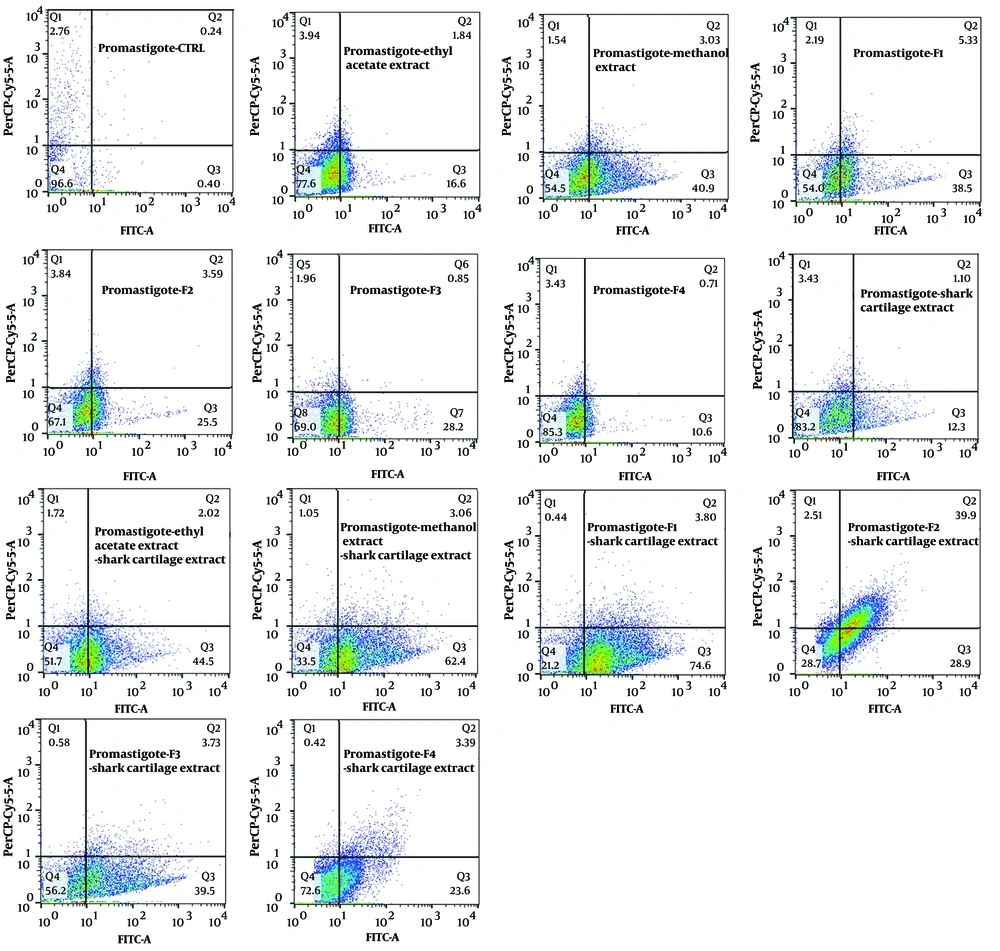
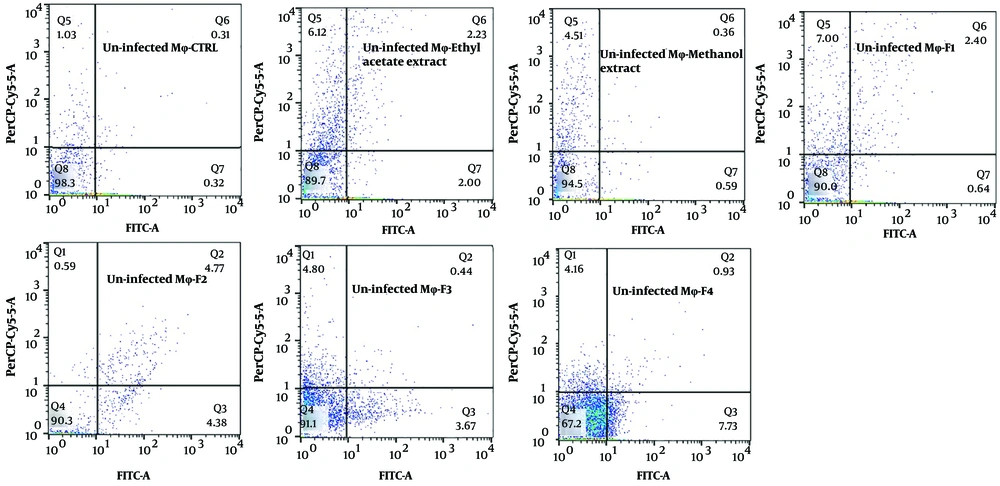
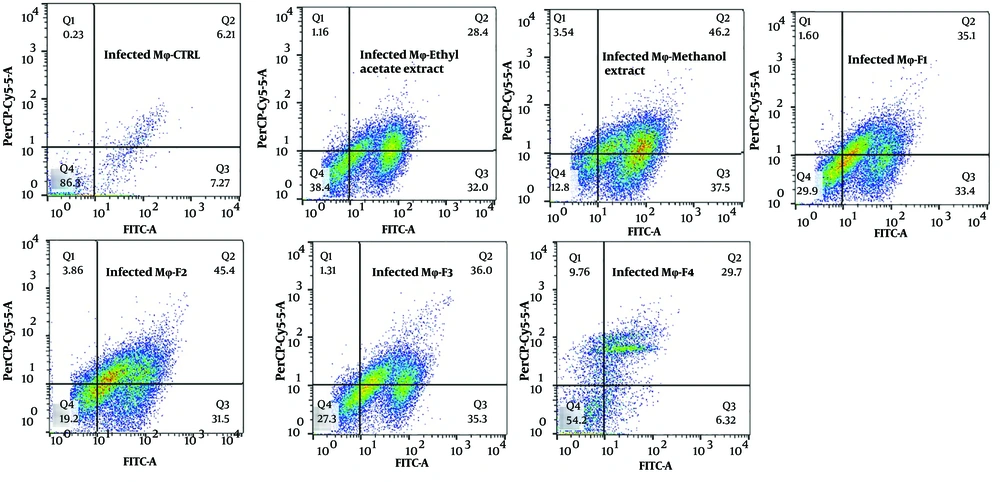
The exposure of stationary-phase L. major promastigotes, as well as infected and non-infected macrophages, to the extracts and fractions of F. tabasensis at their IC50 concentrations after 72 hours induced early apoptosis (Q3, positive for Annexin V), late apoptosis (Q2, positive for Annexin V and PI), necrosis (Q1, positive for PI), and alive cells (Q4, negative for Annexin V and PI) compared to the untreated control cells. The percentage of apoptotic and necrotic cells varied depending on the extract or fraction.
As shown in Figure 2 - 4, significant early apoptosis of promastigote cells was detected in exposure to the methanol extract and F1 fraction. Additionally, the results of flow cytometry indicated the synergistic effects of extracts and fractions when combined with ShCE. Interestingly, the percentage of apoptosis (early and late apoptosis) was higher in the infected macrophage cells compared to the promastigotes and non-infected macrophages. There were significant differences in terms of early and late apoptosis and also necrosis of all extracts and fractions only or combined with ShCE compared to the negative control (CTRL) (P < 0.001).
5. Discussion
The results of the present study revealed that the ethyl acetate and methanol extracts and F1-F4 fractions of F. tabasensis had potential activities, alone or synergistically with ShCE, against both L. major promastigotes and intra-macrophage amastigotes in a dose-dependent manner. The IC50 values for the promastigotes were 3.8 ± 1.13, 2.9 ± 0.55, 2.4 ± 0.29, 4.85 ± 1.2, 4.27 ± 1.82, and 33.5 ± 2.66 µg/mL in the ethyl acetate and methanol extracts and F1-F4 fractions, respectively. The corresponding values for GLU and AmB, as the reference drugs, were 420 ± 1.9 and 33.9 ± 5.1 µg/mL, respectively.
Since macrophage cells are the main host cells for Leishmania parasites, one of the most important steps in the fight against Leishmania parasites is to prevent the infectivity of macrophage cells and destroy them (29). The present findings showed the inhibitory effects of all extracts and fractions against intracellular amastigotes of L. major as the main causative agent of Old World CL. The IC50 value for amastigotes was lower than that of promastigotes (except F2). Based on the results, the combination of ShCE at 200 µg/mL with Ferula extracts and fractions at IC50 concentrations was more efficient than either of the drugs used alone (P < 0.001). However, the toxicity of non-infected macrophage cells was low, and the SI values were > 10 (except F2), representing a safety index for the application of these extracts and fractions to eliminate infected macrophages.
A wide range of antimicrobial (7, 13, 30-33), anthelminthic (34-38), and antiprotozoal (7, 13, 15, 33, 38-40) effects have been attributed to Ferula species. The antileishmanial effects of various oils, extracts, and fractions of the genus Ferula have been examined in previous studies (7, 13, 31, 33, 41, 42). Our findings are in agreement with the results reported by Vahdani et al., which indicated the high in vitro activity of F. assa-foetida ethanol extract (IC50 = 2 ± 0.12, ID50 = 0.65 ± 0.02 µg/mL) against promastigotes and amastigotes of L. major, respectively (41). In another study by these researchers, the aqueous extract of F. assa-foetida exhibited high efficacy against L. major promastigotes (IC50 = 3.6 µg/mL) (41).
Additionally, Bafghi et al. reported the significant preventive effects (> 90%) of F. assa-foetida (oleo-gum resin) on stationary- and logarithmic-phase L. major, using the slide method after 72 hours. Also, the viability of parasites significantly decreased in both growth phases using all drug concentrations compared to the control (43). Moreover, Mahmoudvand et al. found the presence of myrtenal, linalool, terpinolene, terpinen-4-ol, and β-phellandrene in the essential oil of F. macrecolea and reported its great antileishmanial effects in vitro (13). Besides, Andrade et al. reported the slight effects of F. galbaniflua essential oil on L. amazonensis promastigotes and brine shrimp (IC50 = 95.70 ± 1.82 µg/mL and CC50 = 377.26 ± 2.71 µg/mL, respectively) (44).
Recently, Mahmoudvand et al. observed the potential leishmanicidal effects of F. macrecolea essential oils and terpinolene against the promastigotes and amastigotes of L. tropica. The IC50 values of F. macrecolea essential oil and terpinolene against promastigotes were 27.6 and 11.6 µg/mL, respectively. However, their IC50 values against amastigotes were 42.3 and 19.6 µg/mL, respectively. The CC50 values of their compounds were also 471.3 and 207.3 µg/mL for the essential oil and terpinolene, respectively (13). Our results are consistent with their findings of both promastigote and amastigote assays. Moreover, in a study by Mohammadhosseini et al., the antileishmanial activities of three new compounds of the genus Ferula, including fnarthexone, fnarthexol, and conferol, were discussed, and the moderate activities of fnarthexone and fnarthexol with IC50 values of 43.77 ± 0.56 and 46.81 ± 0.81 µg/mL, respectively, were reported. However, the greatest antileishmanial activity, with the highest IC50 value, was attributed to conferol (11.51 ± 0.09 µg/mL) (45).
Generally, there are very few studies on the chemical composition of F. tabasensis. In a study by Bigdeli et al., the compounds of the Iranian Ferula genus were investigated. The bioactive and major compounds, as well as their biological activities, were variable with Ferula species, and volatile sesquiterpenes were the main components of F. tabasensis (46). In another study by Panahi et al., the chemo diversity of volatile compounds of F. tabasensis, along with the other five Ferula species, was determined. Overall, α-pinene, myrcene, thiophene derivatives, sabinene, nonane, octane, β-pinene, and carotol were the major constituents of some Ferula species, especially F. tabasensis (47).
The genus Ferula is mainly characterized by the presence of sesquiterpenes and sesquiterpene coumarins. Meanwhile, the main biological activity of the genus Ferula is ascribed to terpenoid compounds, including monoterpenes, such as α-pinene, β-pinene, myrcene, and limonene, and sesquiterpenes, such as β-caryophyllene, germacrene B, germacrene D, and δ-cadinene (48). It is known that sesquiterpenes and their oxygenated derivatives, alcohols, aliphatic aldehydes, and esters from volatile fractions are the main components of F. tabasensis (7, 46). These compounds lead to the discharge of adenosine triphosphatase and trigger mitochondrial membrane depolarization (49).
The strong antileishmanial activities of sesquiterpenes (50, 51), monoterpenes, sulfur-containing compounds (51), and volatile terpenoids from the genus Ferula (48) have been described in the literature. In our previous research, we found that in vitro exposure of promastigotes to ShCE has significant effects, including a reduction in the growth rate and viability of promastigotes, besides synergistic effects with artemisinin on both promastigotes and amastigotes in vitro and in vivo (21). The present results, for the first time, revealed that both extracts and fractions of F. tabasensis, combined with ShCE, exerted enhanced leishmanicidal effects against L. major.
Flow cytometry is an alternative technique for determining the type of programmed cell death, including early and late apoptosis and necrosis, and also for examining the effects of extracts or fractions on viability or mortality (52). It has been indicated that Leishmania prevents the apoptosis of infected macrophage cells. On the other hand, apoptosis occurs in Leishmania amastigotes and promastigotes following exposure to drugs and herbal extracts (53). In the present study, the results of flow cytometry confirmed the promastigote and amastigote assay results, which suggested significant apoptosis at IC50 concentrations of all extracts and fractions. The percentage of apoptosis (early and late) of promastigotes following exposure to ethyl acetate and methanol extracts and F1-4 fractions of F. tabasensis was measured to be 18.44%, 44.2%, 43.83%, 29.09%, 29.05%, and 11.3%, respectively; these values also increased when the extracts and fractions were combined with ShCE.
In this regard, Gharaei et al. reported the apoptosis-inducing effects of F. gummosa Boiss extracts in AGS, a human adenocarcinoma cell line. In this study, the ethanol extract from plant flowers induced high apoptosis (78%) in the promastigote cells (54). Moreover, in a study by Mousavi et al., the apoptotic effects of auraptene, as one of the key components of 7-prenyloxycoumarins from F. szowitsiana, were documented in the MCF-7 cell line (IC50 = 59.7 µM). In this study, DNA fragmentation was introduced as one of the underlying mechanisms of component-induced apoptosis (55).
5.1. Conclusions
Owing to the potent antileishmanial activity of F. tabasensis extracts and fractions against L. major, especially the methanol extract and F1 fraction used alone or in combination with ShCE, they can be not only introduced as new drug alternatives in antileishmanial therapy, but also support future research for the development of highly effective, affordable, and reliable medicines.