1. Background
Helicobacter pylori is a gram-negative, microaerophilic bacterium. It is responsible for most cases of active chronic gastritis, peptic ulcer, intestinal metaplasia, low-grade mucosa-associated lymphoid tissue lymphoma, and/or cancer development (1). Its infection rate is about 50% worldwide, with prevalence rates ranging from 20% to more than 80% in different countries (2, 3). In gastric mucosal infection cases, eradicating H. pylori seems to treat ulcers and the infection (4, 5). The most effective regimen for the treatment of H. pylori infection is a combination of a proton pump inhibitor and 2 antimicrobial agents (mainly clarithromycin and either metronidazole [MTZ] or amoxicillin) (6).
Different methods are applied to determine the H. pylori susceptibility to MTZ. Nevertheless, an association between MTZ resistance and treatment failure has been found. Combination therapy with clarithromycin and MTZ led to a cure rate of 95% when the strains were susceptible to MTZ and 76% when the strains were MTZ-resistant (MIC = 8 µg/mL, as determined by the agar dilution method recommended by EUCAST) (7, 8).
Metronidazole is one of the well-known compounds of the 5-nitroimidazole class, which has been used as an antimicrobial agent for several years (9). Since derivatives with a 5-nitroimidazole nucleus have been evaluated and characterized for their toxicity and metabolism profile, this nucleus has great potential to be widely applied in drug design (10).
There is a report on the synthesis and biological evaluation of new 5-nitroimidazole derivatives. A group of 1-(2-hydroxypropyl)-2-styryl-5-nitroimidazole derivatives was synthesized, and their antibacterial activities and toxicity were evaluated (11). Metronidazole derivatives that contain piperazine were also introduced. These new compounds exhibited proper antibacterial effects against gram-positive strains (9).
Despite the unique antimicrobial spectrum of 5-nitroimidazole derivatives in the treatment of infectious diseases, especially H. pylori infection, there is still a great concern about H. pylori resistance to MTZ, which would result in the need for new active agents against MTZ-resistant H. pylori strains. These observations have led us to investigate novel MTZ compounds with similar structural properties to overcome the resistance problem.
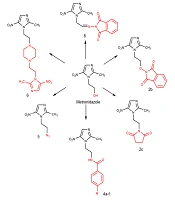
Following our previous research on synthesizing bioactive azole rings (12-20) and considering the biological effects of the nitroimidazole scaffold, this study focused on the design and synthesis of novel compounds containing a nitroimidazole structure. The anti-H. pylori effects of these compounds were evaluated against MTZ-resistant strains (Figure 1).
2. Methods
2.1. Materials
All chemicals and reagents used in this study were commercially attained from Merck or Aldrich Companies (Germany).
For obtaining 1H NMR spectra, a Bruker FT-500 MHz instrument (Bruker Biosciences, USA) was used, and chemical shifts (δ) were reported as parts per million (ppm). The electrospray mass (ESI-MS) spectra of the synthesized compounds and elemental analysis were acquired using an Agilent 4610 triple quadrupole mass spectrometer and elemental analyzer (Costech, Italy), respectively. The melting points of the synthesized compounds were determined using the Electrothermal 9100 melting point apparatus. In addition, the Perkin Elmer 1420 ratio recording spectrometer was used to generate the infrared spectra.
2.2. Synthesis
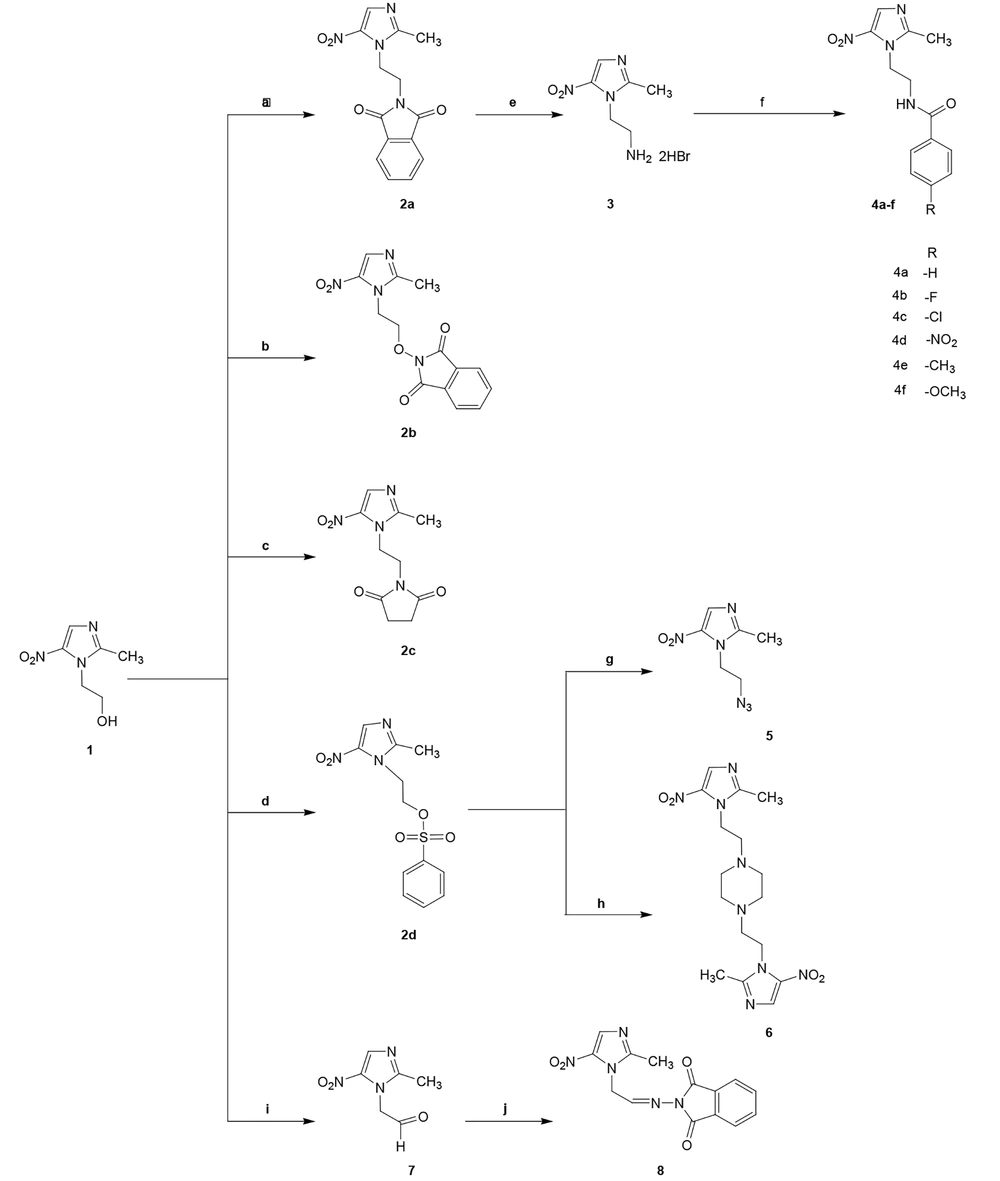
2-(2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethoxy) isoindoline-1,3-dione (2b) (21):
To a mixture of metronidazole 1 (0.5 g, 2.92 mmol), triphenylphosphine (1.125 g, 4.28 mmol), and hydroxyphthalimide (0.7 g, 4.28 mmol) in 26 mL of dry THF, DIAD (diisopropyl azodicarboxylate) (1.9 mL, 9.75 mmol) was added in one portion at room temperature. The reaction mixture was stirred for 24 h. The solvent was removed under low pressure. The residue was triturated with diethyl ether and filtered to obtain a white solid with the following characteristics:
Yield: 77.77%; mp: 184 - 186ºC; crystallization in diethyl ether; IR (KBr, cm-1): 1783, 1734 (C=O),1520 (NO2), 1357 (NO2); 1H NMR (500 MHz, CDCl3): δ: 2.64 (3H, s, CH3), 4.60 (2H, t, J = 4.9 Hz, CH2-N), 4.75 (2H, t, J = 5 Hz, CH2-O), 7.78 (2H, m, ArH), 7.84 (2H, m, ArH), 8.07 (1H, s, Imidazole-H); ESI-MS (m/z): [M+Na]+ 339; Anal. Calc. for C14H12N4O5 (316.08): C 53.17, H 3.82, N 17.71; found: C 53.15, H 3.81, N 17.75.
1-(2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl) pyrrolidine-2,5-dione (2c) (22):
A mixture of metronidazole 1 (0.5 g, 2.92 mmol), triphenylphosphine (1.14 g, 4.34 mmol), and succinimide (0.43 g, 4.34 mmol) in 25 mL dry THF was cooled to 0 - 5ºC. Then, a solution of DIAD (diisopropyl azodicarboxylate) (1.9 mL, 9.75 mmol) in 3 mL dry THF was added dropwise for 20 min. The reaction mixture was stirred for 24 h. The progress of the reaction was monitored by TLC. The solvent was removed to give oil under low pressure. The oil was solidified using diethyl ether and then crystallized from 2-propanol. The following characteristics appeared:
Yield: 55%; mp: 151 - 153ºC; IR (KBr, cm-1): 1703 (C=O), 1528 (NO2), 1374 (NO2); 1H NMR (500 MHz, DMSO-d6): δ: 2.36 (3H, s, CH3), 2.52 (4H, broad s, Succinimide), 3.76 (2H, t, J = 5.9 Hz, CH2-Succinimide), 4.42 (2H, t, J = 5.9 Hz, CH2-Imidazole), 8.00 (1H, s, Imidazole-H); ESI-MS (m/z): [M+Na]+ 275; Anal. Calc. for C10H12N4O4 (252.09): C 47.62, H 4.80, N 22.21; found: C 47.61, H 4.79, N 22.19.
2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl benzenesulfonate (2d):
A solution of metronidazole 1 (10 g, 58.47 mmol) in dry pyridine was cooled in an ice bath; then, benzene sulfonyl chloride (8.1 mL, 63.8 mmol) was added dropwise. The reaction mixture was stirred for 24 h at room temperature. The achieved solution was diluted with ice/water, and the obtained product was washed several times with water, with the following characteristics:
Yield: 83%; mp: 145 - 150 ºC; IR (KBr, cm-1): 1520 (NO2), 1380 (NO2), 1340 (SO2), 1180.
2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethanamine dihydrobromide (3):
A mixture of metronidazole 1 (4 g, 23.4 mmol), triphenylphosphine (9.18 g, 35 mmol), and phthalimide (5.15 g, 35 mmol) in 200 mL dry THF was cooled to 0 - 5ºC. Then, a solution of DIAD (diisopropyl azodicarboxylate) (15.2 mL, 75 mmol) in 20 mL dry THF was added dropwise for 20 min. The reaction mixture was stirred for 1 h. The progress of the reaction was monitored by TLC. The solvent was removed to give oil under low pressure. The oil was solidified using diethyl ether and then crystallized from 2-propanol to obtain 2a. A mixture of 2a (3.02 g, 10 mmol) in 30% HBr (110 mL) was refluxed for 16 h. The acid was separated by distillation. Absolute ethanol (100 mL) was added to the mixture and dried under low pressure. The residue was washed with diethyl ether (4 × 30 mL) and crystallized from ethanol (23). It had the following characteristics:
Yield: 80%; mp: 221 - 228ºC; 1H NMR (500 MHz, DMSO-d6): δ: 2.61 (3H, s, CH3), 3.33 (2H, m, CH2-NH2), 4.60 (2H, t, J = 6.4 Hz, CH2-Imidazole), 8.20 (3H, NH3+), 8.43 (1H, s, Imidazole-H), 9.87 (1H, broad s, NH+-Imidazole); ESI-MS (m/z): [M+H]+ 171; Anal. Calc. for C6H12Br2N4O2 (329.93): C 21.71, H 3.64, N 16.88; found: C 21.73, H 3.65, N 16.91.
2.2.1. General Synthesis Method for Compounds (4a-f)
Five milliliters of triethylamine was added to a solution of 3 (0.5 g) in dry THF (20 mL), and the mixture was cooled to 0 - 5ºC. Then, appropriate benzoyl chloride (2.85 mmol) was added dropwise. The mixture was stirred for 5 h. The solvent was removed under low pressure. The residue was dissolved in dichloromethane (30 mL) and extracted with acidified water by 3 M HCl (5 × 20 mL). The combined aqueous layers were made alkaline (pH 7.5 - 8) with NaOH solution and extracted with dichloromethane (5 × 20 mL). The combined organic layers were washed with a sodium chloride saturated solution (2 × 25 mL) and then dried with Na2SO4. The organic solvent was evaporated under a vacuum. The residue was crystallized from ethanol.
N-(2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl) benzamide (4a) (24)
It had the following characteristics: Yield: 30%; mp: 128.7 - 131.2ºC; IR (KBr, cm-1): 3364 (NH), 1637 (C=O), 1544 (NO2), 1366 (NO2); 1H NMR (500 MHz, CDCl3): δ: 2.52 (3H, s, CH3), 3.83 (2H, m, CH2-NH), 4.63 (2H, t, J = 6.2 Hz, Imidazole-CH2), 6.76 (1H, t, NH), 7.46 (2H, t, J = 7.63 Hz, ArH), 7.55 (1H, t, J = 7.4 Hz, ArH), 7.75 (2H, d, J = 7.3 Hz, ArH), 7.97 (1H, s, Imidazole-H); ESI-MS (m/z): [M+Na]+ 297; Anal. Calc. for C13H14N4O3 (274.11): C 56.93, H 5.14, N 20.43; found: C 56.92, H 5.15, N 20.42.
4-Fluoro-N-(2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethyl)benzamide (4b):
It had the following characteristics: Yield: 35%; mp: 202 - 204ºC; IR (KBr, cm-1); 3386 (NH), 1648 (C=O), 1556 (NO2), 1375 (NO2); 1H NMR (500 MHz, CDCl3): δ: 2.27 (3H, s, CH3), 3.54 (2H, m, CH2-NH), 4.37 (2H, t, J = 6.1 Hz, Imidazole-CH2), 6.89 (2H, t, J = 8.6 Hz, ArH), 7.65 (2H, d, J = 8.6 Hz, ArH), 7.78 (1H, s, Imidazole-H), 8.23 (1H, t, NH); ESI-MS (m/z): [M+Na]+ 315; Anal. Calc. for C13H13FN4O3 (292.10): C 53.42, H 4.48, N 19.17; found: C 53.44, H 4.48, N 19.18.
4-Chloro-N-(2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethyl)benzamide (4c):
It had the following characteristics: Yield: 45%; mp: 197.2 - 199.2ºC; IR (KBr, cm-1): 3348 (NH), 1639 (C=O), 1543 (NO2), 1356 (NO2); 1H NMR (500 MHz, DMSO-d6): δ: 2.35 (3H, s, CH3), 3.63 (2H, m, CH2-NH), 4.44 (2H, t, J = 5.8 Hz, Imidazole-CH2), 7.53 (2H, d, J = 8.8 Hz, ArH), 7.74 (2H, d, J = 7.8 Hz, ArH), 8.02 (1H, s, Imidazole-H), 8.76 (1H, t, J = 5.82 Hz, NH); ESI-MS (m/z): [M+Na]+ 331.5; Anal. Calc. for C13H13ClN4O3 (308.07): C 50.58, H 4.24, N 18.15; found: C 50.57, H 4.24, N 18.16.
N-(2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl)-4- nitrobenzamide (4d):
It had the following characteristics: Yield: 32%; mp: 197.8 - 199.3ºC; IR (KBr, cm-1): 3425 (NH), 1666 (C=O), 1514 (NO2), 1361 (NO2); 1H NMR (500 MHz, DMSO-d6): δ: 2.36 (3H, s, CH3), 3.67 (2H, m, CH2-NH), 4.46 (2H, t, J = 5.7 Hz, Imidazole-CH2), 7.96 (2H, d, J = 8.7 Hz, ArH), 8.05 (1H, t, J = 8.7 Hz, NH), 8.31 (2H, d, J = 8.6 Hz, ArH), 9.02 (1H, s, Imidazole-H); ESI-MS (m/z): [M+H]+ 320, [M+Na]+ 342; Anal. Calc. for C13H13N5O5 (319.09): C 48.90, H 4.10, N 21.94; found: C 49.01, H 4.10, N 21.93.
4-Methyl-N-(2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethyl)benzamide (4e):
It had the following characteristics: Yield: 37%; mp: 187 - 191ºC; IR (KBr, cm-1): 3368 (NH), 1637 (C=O), 1543 (NO2), 1353 (NO2); 1H NMR (500 MHz, DMSO-d6): δ: 2.33 (3H, s, phenyl-CH3), 2.50 (3H, s, Imidazole-CH3), 3.62 (2H, m, CH2-NH), 4.43 (2H, t, J = 5.9 Hz, Imidazole-CH2), 7.25 (2H, d, J = 8.0 Hz, ArH), 7.63 (2H, d, J = 8.1 Hz, ArH), 8.02 (1H, s, Imidazole-H), 8.58 (1H, t, J = 5.3 Hz, NH); ESI-MS (m/z): [M+H]+ 289; Anal. Calc. for C14H16N4O3 (288.12): C 58.32, H 5.59, N 19.43; found: C 58.37, H 5.59, N 19.42.
4-Methoxy-N-(2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethyl)benzamide (4f):
It had the following characteristics: Yield: 25%; mp: 149.2 - 150.3ºC; IR (KBr, cm-1): 3368 (NH), 1642 (C=O), 1523 (NO2), 1370 (NO2); 1H NMR (500 MHz, CDCl3): δ: 2.52 (3H, s, CH3), 3.77 (2H, m, CH2-NH), 3.87 (3H, s, OCH3), 4.62 (2H, t, J = 6.2 Hz, Imidazole-CH2), 6.62 (1H, t, J = 8.8 Hz, NH), 6.95 (2H, d, J = 8.8 Hz, ArH), 7.73 (2H, d, J = 6.9 Hz, ArH), 7.97 (1H, s, Imidazole-H); ESI-MS (m/z): [M+Na]+ 327; Anal. Calc. for C14H16N4O4 (304.12): C 55.26, H 5.30, N 18.41; found: C 55.30, H 5.29, N 18.40.
1-(2-Azidoethyl)-2-methyl-5-nitro-1H-imidazole (5) (25):
A mixture of 2d (1 g, 3.58 mmol), sodium azide (35.8 mmol), triethylamine in water (5 mL), and 18-crown-6-ether in xylene (30 mL) was refluxed for 48 h. The solvent was removed by distillation. The residue was diluted in water and extracted with ethyl acetate (4 × 20 mL). The combined organic layers were evaporated. The residue was triturated with n-hexan. The resulting solid was washed several times with n-hexane, which gave the following characteristics:
Yield: 54%; mp: 50 - 53ºC; IR (KBr, cm-1): 2123 (N3), 1540 (NO2), 1372 (NO2); 1H NMR (500 MHz, DMSO-d6): δ: 2.48 (3H, s, CH3), 3.79 (2H, t, J = 5.6 Hz, CH2-N3), 4.48 (2H, t, J = 5.6 Hz, Imidazole-CH2), 8.03 (1H, s, Imidazole-H); ESI-MS (m/z): [M+H]+ 197; Anal. Calc. for C6H8N6O2 (196.07): C 36.74, H 4.11, N 42.84; found: C 36.65, H 4.12, N 42.87.
1,4-Bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl) piperazine (6):
A mixture of 2d (1 g, 3.58 mmol), piperazine (0.092 g, 1.06 mmol), and sodium bicarbonate (excess) in dioxane was refluxed for 24 h. The solvent was evaporated under low pressure. Water (10 mL) was added to the residue and extracted by chloroform (3 × 10 mL). The combined organic layers were washed with water, dried with Na2SO4, and purified using column chromatography (mobile phase first: chloroform, Second: chloroform 83.3%: methanol 16.6%), giving the following characteristics:
Yield: 25%; mp: 197 - 199ºC; 1H NMR (500 MHz, CDCl3): δ: 2.47 (8H, broad s, Piperazine), 2.53 (6H, s, CH3), 2.67 (4H, t, J = 5.9 Hz, CH2-N), 4.40 (4H, t, J = 5.9 Hz, CH2-Imidazole), 7.94 (2H, s, Imidazole-H); ESI-MS (m/z): [M+Na]+ 415; Anal. Calc. for C16H24N8O4 (392.19): C 48.97, H 6.16, N 28.56; found: C 48.94, H 6.17, N 28.53.
2-(2-Methyl-5-nitro-1H-imidazol-1-yl)acetaldehyde (7):
Oxalyl chloride (2.3 mL, 25.3 mmol) was added dropwise to 160 mL of dry dichloromethane under argon. After that, the reaction mixture was cooled to -50ºC in dry ice. Then, DMSO (17 mL, 240 mmol) was added dropwise. After 20 min, a solution of metronidazole 1 (3 g, 17.54 mmol) in 15 mL DMSO was added dropwise. By passing another 20 min, dry triethylamine (33 mL, 240 mmol) was added dropwise. The reaction was stirred for 10 minutes and then warmed to room temperature. The mixture was diluted in ethyl acetate and extracted with water (3 × 50 mL). The combined aqueous layers were extracted with ethyl acetate (3 × 250 mL). The combined organic layers were washed with saturated NaCl and dried with Na2SO4. The organic solvent was evaporated under a vacuum. The obtained residue was purified using column chromatography (mobile phase: Dichloromethane 97%: Methanol 3%) to obtain the product as an oil (26) with the following characteristics:
Yield: 68%; ESI-MS (m/z): [M+H]+ 170.
2-(2-(2-Methyl-5-nitro-1H-imidazol-1-yl) ethylideneamino)isoindoline-1,3-dione (8):
A mixture of acidified (acetic acid pH = 4 - 4.5) N-aminophthalimide (0.35 g, 2.16 mmol) in dioxane was added dropwise to a solution of 7 (0.37 g, 2.16 mmol) in dioxane at 60ºC, and the reaction mixture was refluxed for 19 h at 60ºC. The solvent was evaporated, and the obtained liquid was triturated with diethyl ether and filtered to obtain a brown solid with the following characteristics:
Yield: 55.2%; mp: 206 - 208ºC, crystallized in diethyl ether; IR (KBr, cm-1): 1785 (C=O), 1734 (C=O), 1533 (NO2), 1358 (NO2); 1H NMR (500 MHz, CDCl3): δ: 2.59 (3H, s, CH3), 5.36 (2H, d, J = 4.0 Hz, CH2-Imidazole), 7.82 (2H, m, ArH), 7.93 (2H, m, ArH), 8.03 (1H, s, Imidazole-H), 9.04 (1H, t, J = 4.0 Hz, HC = N); Anal. Calc. for C14H11N5O4 (313.08): C 53.68, H 3.54, N 22.36; found: C 53.67, H 3.53, N 22.35.
2.3. Biological Activity
2.3.1. Helicobacter pylori Strains
As previously described, 20 clinical isolates recovered from 96 adult patients who were referred to the Digestive Endoscopy Unit of Imam Khomeini Hospital in Tehran, Iran, from 2013 to 2014 were used in this study (8).
2.3.2. Susceptibility Testing
Susceptibility of the bacterial strains was determined based on the minimum inhibitory concentration (MIC) assayed by the agar twofold serial dilutions method according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (7, 8).
The concentrations of metronidazole (MAST, London, United Kingdom) and the tested compounds ranging from 0.016 to 512 μg/mL were made in Muller Hinton agar medium (MHA, 45ºC, Merck, Germany) supplemented with 5% of sheep blood. Five microliters of fresh normal saline suspension with turbidity corresponding to 2 McFarland's standard of each clinically isolated H. pylori strain were inoculated on the agar medium. The inoculated plates were incubated in 5% CO2 for 72 hours at 37ºC (8).
Helicobacter pylori strain RIGLD 245 was used as a reference strain for all the experiments. The MIC was considered the lowest concentration inhibiting the bacterial visible growth. The EUCAST clinical breakpoint of > 8 µg/mL was defined for the metronidazole-resistant strains (7, 8). The results were expressed as the MICs (Table 1). The MIC50 and MIC90, corresponding to the concentrations of the synthesized derivatives inhibiting the 50% and 90% growth of the isolates, respectively, are reported in Table 2.
3. Results and Discussion
3.1. Chemistry
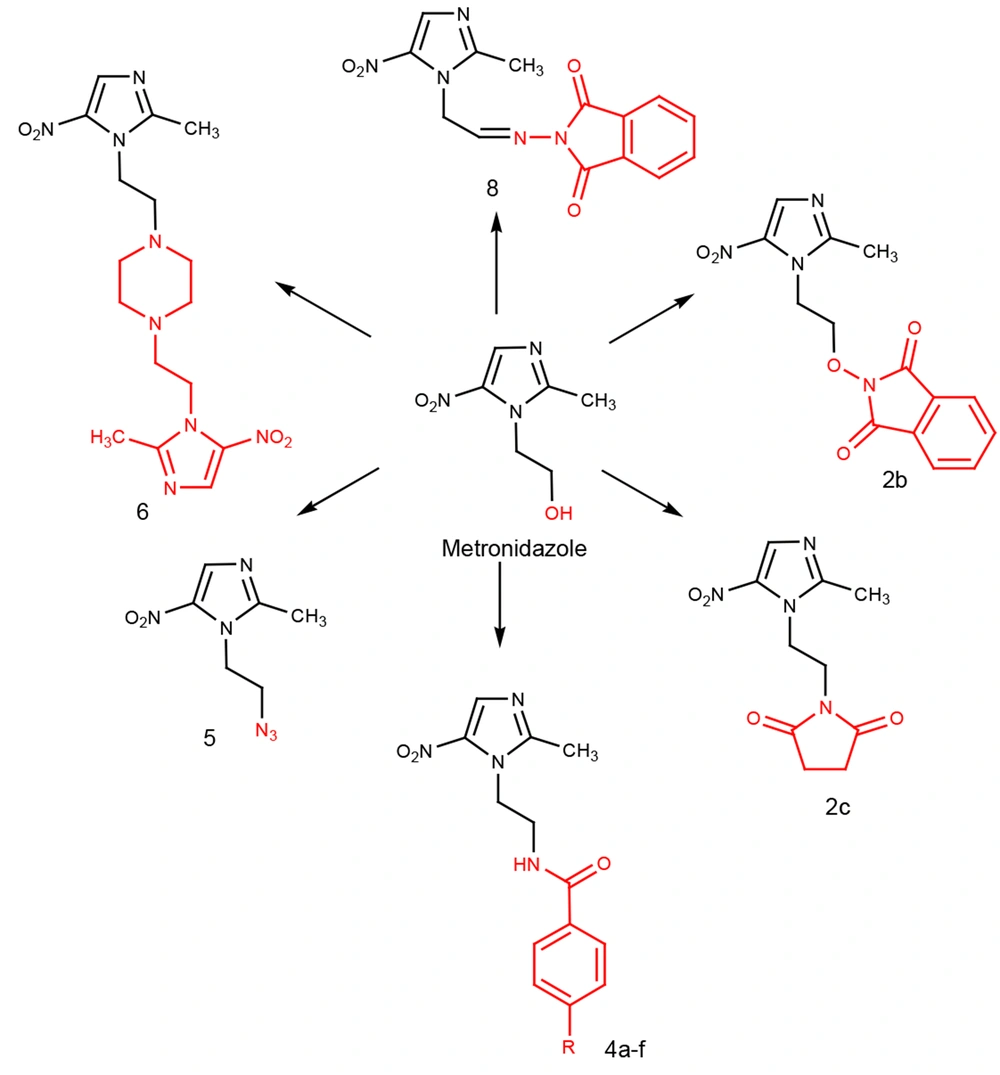
The designed compounds were derived from metronidazole, as shown in Figure 2. Compounds 2a - c were synthesized through the Mitsunobu reaction by metronidazole reacting with three different imides (phthalimide, hydroxy phthalimide, and succinimide) in the presence of triphenylphosphine (PPh3) and diisopropyl azodicarboxylate (DIAD) in dry THF. In all of these reactions, DIAD was added to a mixture of metronidazole, triphenylphosphine, and corresponding imides at 0 - 5ºC, except for hydroxyphthalimide, where DIAD was added to the mixture at room temperature in one part.
Reagents and conditions: A, Phthalimide, PPh3, DIAD, dry THF, Stir at rt; B, Hydroxyphthalimide, PPh3, DIAD, dry THF, Stir at rt; C, Succinimide, PPh3, DIAD, dry THF, Stir at 0ºC; D, Benzenesulfonyl chloride, dry pyridine, stir at rt; E, HBr, Reflux 16 h; F, Benzoyl chloride derivatives, dry THF, Et3N, stir 5 h; G, NaN3, Xylene, crown ether, water, Et3N, reflux 48 h; H, Piperazine, NaHCO3, reflux 24 h; I, Oxalyl chloride, DMSO, Et3N, stir at -50ºC; J, N-aminophthalimide, dioxane, acetic acid, reflux.
Compounds 4a - f were amide derivatives of 5-nitroimidazole and were obtained after a reaction between compound 3 and appropriate benzoyl chloride in dry THF.
The benzene sulfonate derivative of metronidazole (2d) was obtained after a reaction between metronidazole (1) and benzene sulfonyl chloride in dry pyridine. Compound 2d was reacted with sodium azide to obtain compound 5. Crown ether as a phase transfer catalyst was used in this reaction to increase sodium azide's solubility. Compound 6 was obtained by the reaction of 2d with piperazine at a 3.6/1 ratio.
The structures of the synthesized compounds were characterized by IR, 1H-NMR, and ESI-MS.
3.2. Biological Activity
The synthesized compounds were evaluated for their inhibitory activity against 20 clinically isolated H. pylori strains resistant to MTZ by determination of MIC, MIC50, and MIC90. The results are shown in Tables 1 and 2.
| Compounds; Strains | 1 Metronidazole | 2b | 2c | 4a | 4b | 4c | 4d | 4e | 4f | 5 | 6 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 8 | 8 | 8 | 8 | 8 | ND | 8 | 8 | 8 | 8 | ND |
| 2 | 64 | 4 | 4 | 4 | 4 | 4 | ND | 4 | 4 | 4 | 4 | ND |
| 3 | 64 | 8 | 8 | 8 | 8 | 8 | ND | 8 | 8 | 8 | 8 | ND |
| 4 | 64 | 4 | 4 | 4 | 4 | 4 | ND | 4 | 4 | 4 | 4 | ND |
| 5 | 128 | 16 | 32 | 16 | 16 | 16 | ND | 8 | 16 | 8 | 8 | ND |
| 6 | 128 | 16 | 32 | 32 | 16 | 16 | ND | 8 | 16 | 8 | 8 | ND |
| 7 | 256 | 32 | 32 | 32 | 32 | 32 | ND | 16 | 32 | 16 | 16 | ND |
| 8 | 256 | 32 | 32 | 32 | 32 | 32 | ND | 8 | 32 | 8 | 16 | ND |
| 9 | 256 | 32 | 16 | 32 | 8 | 32 | ND | 16 | 32 | 8 | 16 | ND |
| 10 | 256 | 16 | 16 | 16 | 16 | 16 | ND | 8 | 32 | 8 | 8 | ND |
| 11 | 512 | 32 | 32 | 32 | 32 | 32 | ND | 16 | 16 | 16 | 16 | ND |
| 12 | 512 | 32 | 16 | 16 | 32 | 16 | ND | 16 | 16 | 16 | 8 | ND |
| 13 | 512 | 16 | 8 | 32 | 8 | 32 | ND | 8 | 8 | 8 | 8 | ND |
| 14 | 512 | 64 | 16 | 16 | 32 | 32 | ND | 16 | 32 | 16 | 16 | ND |
| 15 | 512 | 16 | 32 | 32 | 32 | 32 | ND | 16 | 32 | 16 | 16 | ND |
| 16 | 512 | 64 | 32 | 32 | 32 | 8 | ND | 16 | 32 | 16 | 16 | ND |
| 17 | 512 | 8 | 8 | 8 | 8 | 8 | ND | 8 | 8 | 16 | 8 | ND |
| 18 | 512 | 32 | 32 | 8 | 32 | 32 | ND | 16 | 32 | 16 | 16 | ND |
| 19 | >512 | 128 | 64 | 64 | 128 | 64 | ND | 32 | 64 | 32 | 32 | ND |
| 20 | >512 | 128 | 64 | 64 | 64 | 64 | ND | 32 | 128 | 32 | 64 | ND |
Abbreviation: ND, not determined.
| Compounds | MIC50 (µg/mL) | MIC90 (µg/mL) |
|---|---|---|
| 1 (metronidazole) | 256 | 512 |
| 2b | 16 | 64 |
| 2c | 16 | 32 |
| 4a | 16 | 32 |
| 4b | 16 | 32 |
| 4c | 16 | 32 |
| 4d | ND | ND |
| 4e | 8 | 16 |
| 4f | 16 | 32 |
| 5 | 8 | 16 |
| 6 | 8 | 16 |
| 8 | ND | ND |
Abbreviation: ND, not determined.
As presented in Tables 1 and 2, almost all synthesized compounds were effective against metronidazole-resistant H. pylori strains. The para methyl derivative (4e) was identified as the most active compound (MIC50 = 8 μg/mL) against metronidazole-resistant H. pylori strains amongst benzoyl metronidazole derivatives (4a-f). Compounds 5 and 6 with azide and piperazine substituents exerted the highest inhibitory activity against metronidazole-resistant H. pylori strains. The obtained MIC90 values of these compounds were 16 μg/mL, which is 32-fold over the metronidazole.
4. Conclusions
Some nitroimidazole analogs were synthesized, and their inhibitory activity against 20 clinically isolated H. pylori strains resistant to metronidazole was evaluated by determining MIC50 and MIC90. Compounds 4e, 5, and 6 were the most active compounds, with the MIC90 of 8 μg/mL and MIC90 of 16 μg/mL.