1. Background
The Coleus amboinicus plant, known as Lour, holds significant potential as a source of food and medicinal ingredients. Among the Batak tribe, it is referred to as bangun-bangun leaves and is commonly consumed as a vegetable. Beyond its nutritional value, C. amboinicus is rich in secondary metabolite compounds, making it a promising candidate for pharmaceutical applications. These compounds include phenolics, polyphenols, flavonoids, diterpenes, coumarins, diterpenoids, and alkaloids (1, 2), which have demonstrated potential as antioxidants (3, 4), anticancer agents, antiproliferatives, and cytotoxics (5).
Cancer ranks among the top three causes of death worldwide for both men and women, with breast cancer being the leading cause of death among women, and lung cancer being the second leading cause for both genders. Conventional treatments for these cancers typically involve chemotherapy, surgery, radiation, targeted therapy, and immunotherapy (6, 7). However, current chemotherapy regimens, which often employ combinations of drugs acting through different mechanistic pathways on tumor cells, have shown limited efficacy and are associated with significant side effects, including drug resistance and toxicity. This can lead to a decrease in quality of life and survival rates (8). Similarly, radiation therapy, although used in conjunction with chemotherapy, can have severe side effects. The use of radiation in thoracic therapy can lead to a range of complications, such as restrictive cardiomyopathy, coronary artery disease, pericardial disease, and heart failure with either preserved or reduced ejection fraction (9, 10).
The current treatment methods for lung and breast cancer are often found to be ineffective. As a result, there is a significant interest in exploring the potential of active metabolic compound derivatives (bioactive compounds) extracted from C. amboinicus leaves for treating these cancers. LC/HRMS offers a promising approach for the discovery of detailed metabolites and metabolomics by detecting both primary and minor constituents in natural extracts. This technology provides high sensitivity and efficiency, enabling the identification and confirmation of the chemical structures of metabolite compounds in plants. The efficacy of this plant species in various treatments has been documented (11).
Utilizing active compounds from plants is anticipated to benefit 50 – 60% of cancer patients, offering an alternative therapy for the treatment of various cancers and other diseases (12). The adoption of natural agents and their derivatives is seen as a superior method for disease prevention and treatment (13).
2. Objectives
Given this context, the current study aims to evaluate the potential activity of C. amboinicus leaf extract against breast cancer cells (MCF-7) and lung cancer cells (A549) using the MTT assay and to identify potential metabolite content with LC/HRMS instruments.
3. Methods
3.1. Materials
The study utilized C. amboinicus leaves, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), Dimethyl sulfoxide (DMSO) from Merck (D1435), Trypsin-EDTA, Elisa microplate readers, ethanol, n-hexane, chloroform, ethyl acetate (all pro analysis grade), cisplatin (EDQM C22100000), doxorubicin, antibiotics (Sigma), 96-well plates (Merk Nest 701001), Phosphate Buffered Saline (PBS) (Gibco 18912-014), PrestoBlue™ Cell Viability Reagent (Thermofisher A132), Roswell Park Memorial Institute (RPMI-1640) and DMEM-H medium (Gibco 11875-093), Fetal Bovine Serum (FBS) (Gibco 10270-106).
3.2. Preparation and Extraction of C. amboinicus Leaves
Coleus amboinicus was sourced from Sosor Ladang Village, Toba Regency, North Sumatra Province, Indonesia, and authenticated by a botanist (voucher number 0220/S.Tb./I/2023). Fresh green leaves were cleaned under running water, air-dried, and subsequently dried in an oven at 50°C. The dried leaves were ground into a powder. Ethanol was used as the solvent for the maceration extraction over three days. The maceration filtrate was strained using Whatman No. 1 filter paper. The marc was re-macerated under identical conditions. The ethanol extract of C. amboinicus was concentrated under reduced pressure using a rotary evaporator at 55°C. Part of this concentrated ethanol extract underwent further partitioning with solvents of varying polarities in a separating funnel, starting with n-hexane, followed by chloroform, and finally ethyl acetate. This process yielded initial ethanol extract, n-hexane extract, chloroform extract, ethyl acetate extract, and the remaining ethanol extract, which were utilized in subsequent stages (3, 4).
3.3. Phytochemical Screening
Phytochemical screening was conducted on the five extracts utilizing standard reagents. This screening aimed to qualitatively identify the presence of bioactive compounds in each extract, including phenolics and polyphenols, steroids, triterpenoids, saponins, and alkaloids (14, 15).
3.4. Metabolomics Preparation and Analysis
Fifty milligrams of each dry extract (initial ethanol, n-hexane, chloroform, ethyl acetate, and residual ethanol) were placed into 2 mL centrifuge tubes. One milliliter of LC-MS-grade methanol was added to each tube, followed by vortexing for 1 minute. The samples were then centrifuged at 5000 rpm for 10 minutes. The analysis was performed using liquid chromatography and high-resolution orbitrap spectrometry, adopting methods from prior research. The chromatography column measured 100 mm x 2.1 mm ID x 2.6 µm. The mobile phase consisted of water with 0.1% formic acid (A) and ethanol with 0.1% formic acid (B), both of MS-grade, at a flow rate of 0.3 mL/minute. Untargeted metabolomic compound screening for each extract was performed using full MS/dd-MS^2 acquisition mode in either positive or negative polarity/ionization states (16, 17).
3.5. Preparation and Testing of Anticancer Activity in Vitro
3.5.1. Cell Culture
Breast cancer cells (MCF-7) and lung cancer cells (A549) were maintained at the Integrated Laboratory of Padjadjaran University, Bandung, Indonesia. The RPMI and DMEM-H culture media were prepared, containing 10% PBS and 50 µm/50 mL antibiotics. The cell cultures were incubated at 37°C and 5% CO2 until they reached a minimum growth percentage of 70%.
3.5.2. Cytotoxicity Tests Against Cancer Cells
The cultured MCF-7 and A549 cells were advanced to the cytotoxicity testing stage, utilizing various concentrations of each extract (7.81; 15.63; 31.25; 62.5; 125; 250; 500; and 1000 µg/mL) dissolved in DMSO. Cisplatin was employed as the positive control for breast cancer cell testing, and doxorubicin for lung cancer cells. The methodology was based on procedures outlined in prior research (18, 19).
4. Results and Discussion
4.1. Phytochemical Screening
A total of 1.14 kg of C. amboinicus leaf powder was extracted using ethanol, yielding 108.01 ± 0.01 g of concentrated ethanol extract. Phytochemical screening with standard reagents provided qualitative insights into the bioactive compounds present in each extract (Table 1).
| No | Phytochemical Components | Reagent | Initial Ethanol Extract | Extract Partition | |||
|---|---|---|---|---|---|---|---|
| n-Hexane | Chloroform | Ethyl Acetate | Residual Ethanol | ||||
| 1 | Flavonoids | Shinoda test | + | - | - | + | + |
| 2 | Phenolics and polyphenolics | FeCl3 5% in ethanol | + | - | + | + | + |
| 3 | Alkaloids | Dragendroff | + | - | + | - | + |
| Mayer | + | - | + | - | + | ||
| Wagner | + | - | - | - | + | ||
| 4 | Triterpenoids/steroids | Liebermann-Buchard | + | + | + | + | + |
| 5 | Saponins | Foaming test | + | + | + | - | + |
Results of Phytochemical Screening of C. amboinicus Extract
The presence of diverse bioactive compounds in C. amboinicus leaf extract, including alkaloids, diterpenes, triterpenes, and polyphenols, suggests potential pharmacological activities (1, 20). These secondary metabolite compounds, such as alkaloids, diterpenes, triterpenes, and polyphenols have shown anticancer potential in various studies and are utilized therapeutically (21). The diverse secondary metabolites in C. amboinicus leaf extract correlate positively with its potential anticancer properties.
4.2. Metabolomic Analysis of C. amboinicus Extract Using LC/HRMS
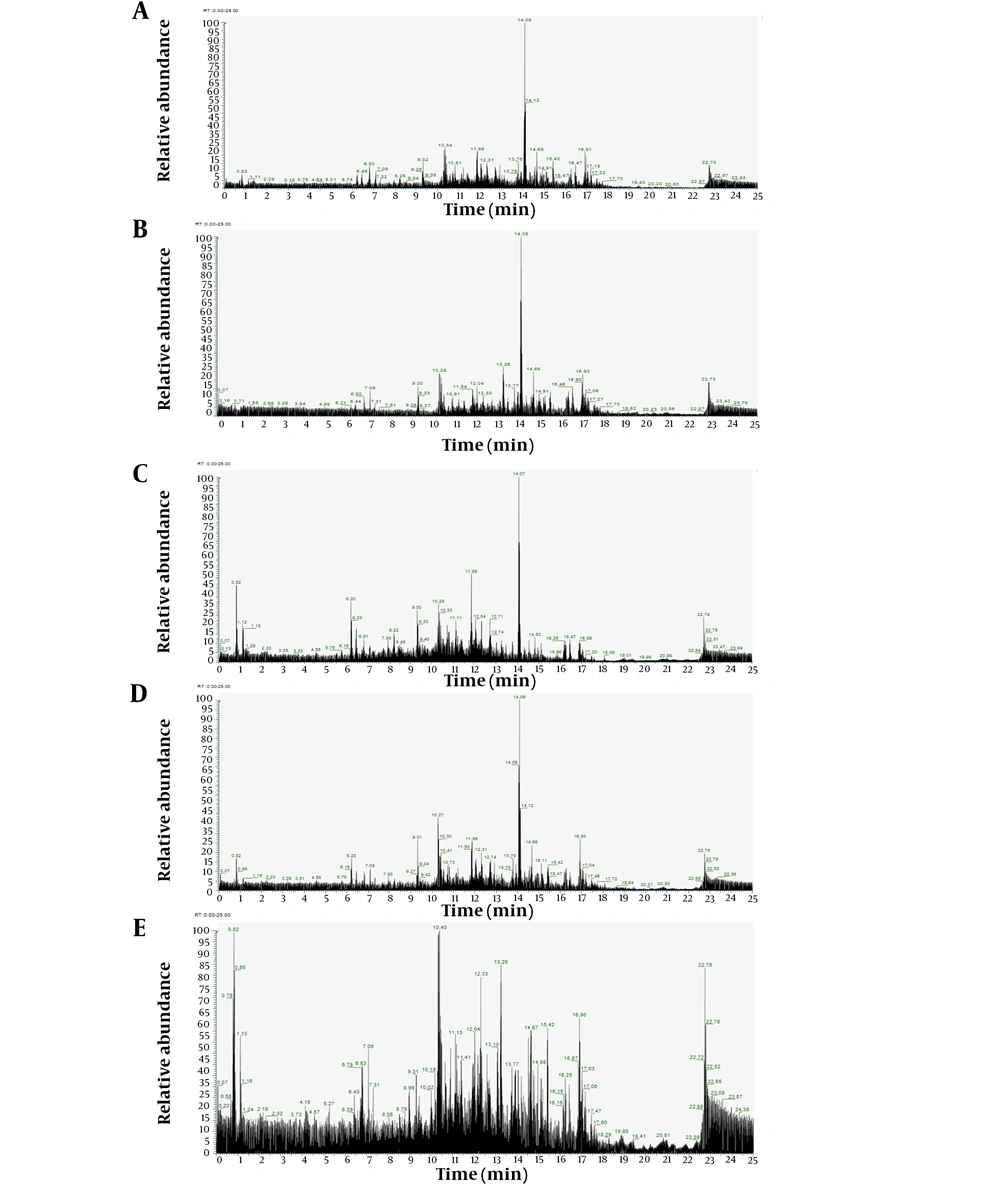
The LC/HRMS analysis employed the Ultimate 3000 RSLC system coupled with a Fusion Lumos Tribrid orbital mass spectrometer. Compounds were ionized using a heated electrospray ionization source in positive mode. A comprehensive MS spectrum was acquired on the Orbitrap at a resolution of 120,000, spanning a mass range of m/z 160 - 2000. The four most intense ions from the full scan, within the mass range of m/z 400 - 2000, underwent fragmentation both in collision-induced dissociation (CID) mode and higher-energy C-trap dissociation (HCD) mode. This approach enhances the capability to analyze complex sample matrices, achieving separations 5 - 10 times more rapidly than conventional methods (22, 23). Utilizing the LC/HRMS instrument represents an advanced method with heightened sensitivity and efficiency. Furthermore, it facilitates the identification of a detailed metabolomic profile, including both primary and minor constituents in natural extracts. The metabolomic profile of each C. amboinicus extract via LC/HRMS is novel information, crucial for advancing research. The chromatogram analysis results for each C. amboinicus leaf extract using LC/HRMS are presented in Figure 1.
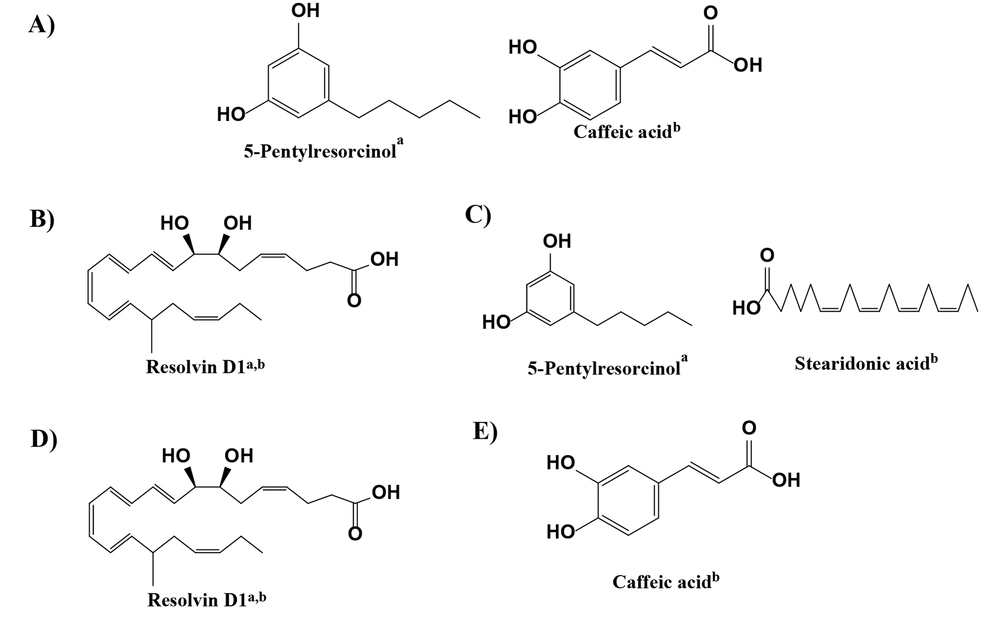
The LC/HRMS analysis results for the initial ethanol extract identified 10 compounds based on their peak areas, as detailed in Table 2. The top three constituents of the initial ethanol extract, from a total of 284 identified components, were: (1) 5,6-dihydroxy-2,6-bis(3-methyl-2-buten-1-yl)-4-(4-methylpentanoyl)-4-cyclohexene-1,3-dione (C22H32O5; 35.706%), (2) gibberellin A24 (C20H26O5; 10.024%), and (3) NP-020713 (C20H26O4; 4.096%). The LC/HRMS analysis of the extracts obtained from partitioning the initial ethanol extract revealed the top 10 compounds by peak area for each extract (Table 3). For the n-hexane extract, from 210 identified components, the top three were: (1) (4S,5E)-4-Hydroxy-6-{(1S,2R,5S)-5-hydroxy-3-oxo-2-[(2Z,5Z,8Z)-2,5,8-undecatrien-1-yl]cyclopentyl}-5-hexenoic acid (C22H32O5; 48.466%), (2) 1-(4-Hydroxy-3-methoxyphenyl)-3,5-decanediyl diacetate (C21H32O6; 7.177%), and (3) 5-Pentylresorcinol (C11H16O2; 3.464%). For the chloroform extract, out of 325 identified components, the leading three were: (1) resolvin D1 (C22H32O5; 13.485%), (2) 5-Pentylresorcinol (C11H16O2; 6.887%), and (3) NP-005870 (C20H26O5; 5.892%). The ethyl acetate extract, with 264 identified components, was dominated by: (1) Resolvin D1 (C22H32O5; 40.878%), (2) gibberellin A24 (C20H26O5; 7.239%), and (3) NP-020713 (C20H26O4; 4.927%). For the residual ethanol extract, from 293 identified components, the top three were: (1) choline (C5H13NO; 6.639%), (2) 9-HOTE (C18H30O3; 5.224%), and (3) (1Z)-1-(4-Hydroxy-3-methoxyphenyl)-1-dodecene-3,5-dione (C19H26O4; 3.653%).
| No | Initial Ethanol Extract | |||
|---|---|---|---|---|
| Compounds | Formula | Group Area | Ionization (ESI) | |
| 1 | 5,6-Dihydroxy-2,6-bis(3-methyl-2-buten-1-yl)-4-(4-methylpentanoyl)-4-cyclohexene-1,3-dione | C22H32O5 | 19497374364 | - |
| 2 | gibberellin A24 | C20H26O5 | 5473876018 | - |
| 3 | NP-020713 | C20H26O4 | 2236370277 | + |
| 4 | NP-005870 | C20H26O5 | 2005457620 | - |
| 5 | 5-Pentylresorcinol | C11H16O2 | 1835585634 | + |
| 6 | Arachidonic acid | C20H32O2 | 1764346397 | + |
| 7 | 13(S)-HOTrE | C18H30O3 | 1452905769 | - |
| 8 | haplophytine | C37H40N4O7 | 1024190439 | + |
| 9 | 2-Hydroxy-3-(phosphonooxy)propyl (9Z,12Z,15Z)-9,12,15-octadecatrienoate | C21H37O7P | 745059283 | - |
| 10 | 9S,13R-12-Oxophytodienoic acid | C18H28O3 | 603455078 | + |
Metabolomics Components of the Initial Ethanol Extract with the Highest Peak Area
| No | n-Hexane Extract | Chloroform Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | Formula | Group Area | Ionization (ESI) | Compounds | Formula | Group Area | Ionization (ESI) | |
| 1. | (4S,5E)-4-Hydroxy-6-{(1S,2R,5S)-5-hydroxy-3-oxo-2-[(2Z,5Z,8Z)-2,5,8-undecatrien-1-yl]cyclopentyl}-5-hexenoic acid | C22H32O5 | 20872483347 | + | Resolvin D1 | C22H32O5 | 6324859959 | + |
| 2. | 1-(4-Hydroxy-3-methoxyphenyl)-3,5-decanediyl diacetate | C21H32O6 | 3090632646 | - | 5-Pentylresorcinol | C11H16O2 | 3230461664 | + |
| 3 | 5-Pentylresorcinol | C11H16O2 | 1491643393 | + | NP-005870 | C20H26O5 | 2763718431 | - |
| 4 | haplophytine | C37H40N4O7 | 1471009433 | + | NP-020713 | C20H26O4 | 2436462762 | + |
| 5 | Arachidonic acid | C20H32O2 | 960872349 | + | 13(S)-HOTrE | C18H30O3 | 1659921023 | - |
| 6 | Pheophorbide A | C35H36N4O5 | 900790852 | + | Stearidonic acid | C18H28O2 | 1524021068 | + |
| 7 | 9-HOTE | C18H30O3 | 810001605 | - | 4-Indolecarbaldehyde | C9H7NO | 1353428049 | + |
| 8 | Stearidonic acid | C18H28O2 | 627201677 | + | NP-017061 | C20H30O4 | 1330723133 | + |
| 9 | Linolenic acid ethyl ester | C20H34O2 | 612428711 | + | NP-005870 | C20H26O5 | 1006625676 | - |
| 10 | α-Linolenic acid | C18H30O2 | 556204914 | + | Paracetamol | C8H9NO2 | 774182422 | + |
| Ethyl acetate extract | Residual ethanol extract | |||||||
| 1. | Resolvin D1 | C22H32O5 | 19855216920 | + | Choline | C5H13NO | 2066589120 | + |
| 2. | gibberellin A24 | C20H26O5 | 3516227224 | - | 9-HOTE | C18H30O3 | 1626215750 | - |
| 3 | NP-020713 | C20H26O4 | 2393343023 | + | (1Z)-1-(4-Hydroxy-3-methoxyphenyl)-1-dodecene-3,5-dione | C19H26O4 | 1137046653 | - |
| 4 | Hexyl 2-furoate | C11H16O3 | 1927943357 | + | 3,3'-Diisopropyl-6,6'-dimethyl-2,2',5,5'-biphenyltetrol | C20H26O4 | 1065773928 | - |
| 5 | 13(S)-HOTrE | C18H30O3 | 1803807869 | - | 1,2,3,4-Tetramethyl-1,3-cyclopentadiene | C9H14 | 972978432 | + |
| 6 | NP-005870 | C20H26O5 | 1604081151 | - | 6-Hydroxy-3,4a,5-trimethyl-2-oxo-2,4,4a,5,6,7,8,8a,9,9a-decahydronaphtho[2,3-b]furan-4-yl (2Z)-2-methyl-2-butenoate | C20H28O5 | 923282617 | - |
| 7 | Arachidonic acid | C20H32O2 | 948801732 | + | 2-Hydroxy-3-(phosphonooxy)propyl (9Z,12Z,15Z)-9,12,15-octadecatrienoate | C21H37O7P | 810505519 | - |
| 8 | 4-Indolecarbaldehyde | C9H7NO | 553570860 | + | Caffeic acid | C9H8O4 | 708108701 | - |
| 9 | 8-{3-Oxo-2-[(2E)-2-penten-1-yl]-1-cyclopenten-1-yl}octanoic acid | C18H28O3 | 540453342 | + | 3,4-Methylenesebacic acid | C12H18O4 | 677578291 | - |
| 10 | Asiatic acid | C30H48O5 | 505413139 | - | Arachidonic acid | C20H32O2 | 573104530 | + |
Metabolomics of Each Extract Resulting from Partitioning from Ethanol Extract Based on the Highest Peak Area
4.3. Cytotoxicity of C. amboinicus Leaf Extract
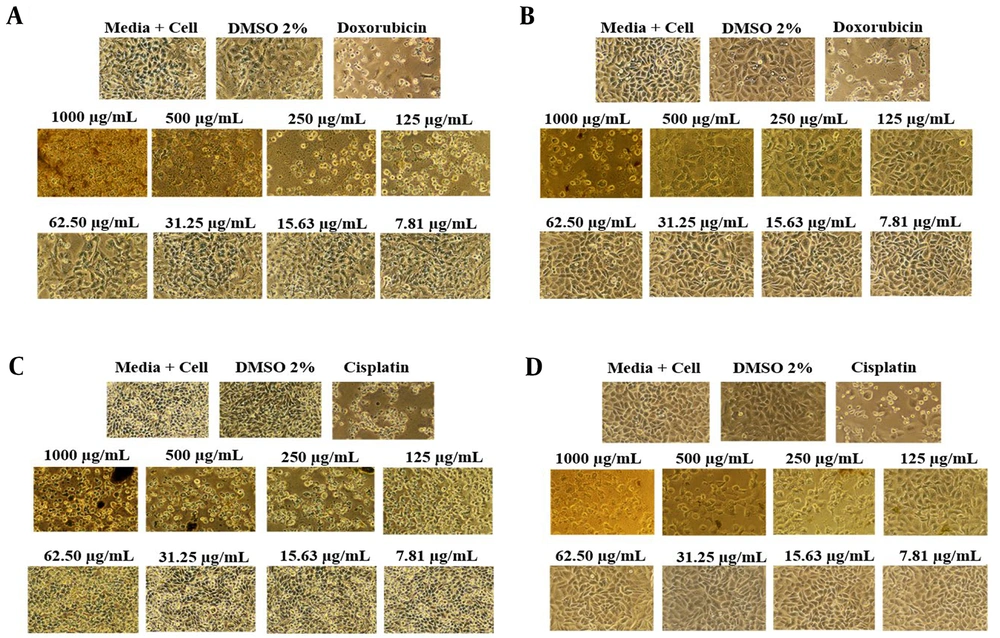
The anticancer activity of each extract was assessed using the MTT assay, a colorimetric technique evaluating mitochondrial cell dehydrogenase enzyme activity. In this assay, the conversion of tetrazole (yellow) to formazan (purple) occurs in living cells, indicating metabolic activity. If the cells are deceased, the enzymatic reaction does not proceed, and the solution remains yellow (24). The anticancer efficacy of each extract was tested in vitro on lung cancer cells (A549) and breast cancer cells (MCF-7). The efficacy of each extract against these cancer cell types is summarized in Table 4.
| No | Treatment | IC50, µg/mL | ||
|---|---|---|---|---|
| Lung Cancer Cells (A549) | Breast Cancer Cells (MCF-7) | Normal Cells (CV-1) | ||
| 1. | Initial ethanol extract | 212.80 | 154.80 | 501.19 |
| 2. | n-hexane extract | 544.80 | 217.20 | 630.96 |
| 3. | Chloroform extract | 251.20 | 102.30 | 189.10 |
| 4. | Ethyl acetate extract | 554.60 | 108.00 | 457.09 |
| 5. | Residual ethanol extract | 554.10 | 396.40 | 531.00 |
| 6. | Cisplatin | - | 53 a | - |
| 7. | Doxorubicin | 2.1 a | - | - |
Testing the Anticancer Activity of C. amboinicus Leave Extract Using the MTT Method
According to Table 4, the initial ethanol extract exhibits more potent anticancer activity (IC50) against MCF-7 cells (154.80 µg/mL) compared to A549 cells (212.80 µg/mL) and displays lower cytotoxicity towards normal cells (501.19 µg/mL) (Figure 2). The examination of extracts derived from partitioning the initial ethanol extract revealed that the anticancer activity in MCF-7 cells was more pronounced than in A549 cells, with lower cytotoxic effects on normal cells, except for the chloroform extract's cytotoxic properties. As per the National Cancer Institute (NCI) classification of cytotoxic activity values for the initial ethanol extract, the chloroform extract falls into the weak category, whereas the n-hexane, ethyl acetate, and residual ethanol extracts are considered inactive against lung cancer cells (A549). In contrast, all extracts exhibit weak anticancer activity against breast cancer cells (MCF-7) and are non-cytotoxic to normal cells, except for the chloroform and ethyl acetate extracts. The NCI categorizes cytotoxic activity based on the IC50 against cancer cells as follows: IC50 < 20 μg/mL is classified as high, IC50 20 - 100 μg/mL as medium, IC50 101 - 500 μg/mL as weak, and IC50 > 500 μg/mL as inactive regarding anticancer potential (25). Metabolomic compounds with the largest peak area are directly associated with the anticancer activity against breast (MCF-7) and lung cancer (A549) for each extract (Figure 3).
4.4. Conclusions
The C. amboinicus leaf extract is rich in various metabolites, including phenolic and polyphenol groups, flavonoids, alkaloids, steroids, triterpenoids, and saponins. Furthermore, this plant extract demonstrates potential anticancer activity against lung (A549) and breast (MCF-7) cancer cells. Notably, the initial ethanol extract showed the most potent activity against lung cancer cells (A549) with an IC50 value of 212.80 µg/mL, while the chloroform extract, obtained through sequential partitioning, was most effective against breast cancer cells (MCF-7) with an IC50 value of 102.30 µg/mL. Further isolation and testing are essential to identify active compounds effective against lung (A549) and breast (MCF-7) cancer cells.