1. Background
Ovarian cancer is a type of solid tumor that primarily affects women, particularly those who are postmenopausal. It ranks as the sixth most common cancer among women and is the fifth leading cause of cancer-related deaths (1). Initial treatments are ineffective for 20 - 30% of patients due to resistance to single-drug therapy (2). Therefore, it is recommended to use combinations of commonly prescribed drugs, such as carboplatin, with other medications like liposomal doxorubicin, gemcitabine, or taxanes in higher doses to prevent cancer recurrence (3). Furthermore, high doses of these drugs, such as cisplatin used in resistant cases, can lead to various side effects, including nausea and drug intolerance, as well as damage to vital body tissues such as the kidneys, heart, liver, and nervous system.
Researchers have focused on macrocyclic diterpene compounds called myrsinanes derived from Euphorbia species, studying their potential anti-cancer properties and effectiveness against multi-drug resistance. These compounds can be categorized into premyrsinanes, myrsinanes, and cyclomyrsinanes. They are biochemically synthesized from jatrophanes and lathyranes (4, 5). These specialized polyoxygenated and polyester compounds are not widely distributed in nature but are commonly found in the Euphorbiaceae family, underscoring the biogenetic significance of this plant family in isolating these compounds. Myrsinanes have demonstrated strong cytotoxic effects against phytohemagglutinin-activated T-cell proliferation but only moderate activity against solid cancer cells (4, 5).
In this family, Euphorbia malleata is found in Iran's central plateau region within the provinces of Isfahan and Yazd. This plant mainly grows on dry, rocky slopes and foothills at altitudes ranging from 190 to 320 meters (6). In previous studies, researchers identified one triterpene, oleanolic acid; three simple phenolics, methyl gallate, scopoletin, and catechin; and two flavonoids, hyperoside and 5,7,3′,5′-tetrahydroxyflavanone in this plant (7).
2. Objectives
The objective of this study was to isolate polyester macrocyclic diterpenoids and to assess the cytotoxic effects of the isolated diterpenes on both wild-type and resistant A2780 ovarian cancer cells. Additionally, the study evaluated combination indices for their use in combination treatment with cisplatin.
3. Methods
3.1. Plant Material
Aerial parts of Euphorbia malleata Boiss., also known by the synonym Tithymalus malleatus (Boiss.) Soják, were collected in July 2017 during flowering from Taragh-Roud, Natanz County, Isfahan, Iran. The aerial parts were dried in shaded conditions at 25°C. The plant was identified by Joharchi (6), and a voucher specimen numbered SAM-3393 is kept in the Samsam-Shariat Herbarium of the Department of Pharmacognosy at Isfahan University of Medical Sciences.
3.2. Extraction and Isolation
After drying, the plant material was powdered (5 kg) and extracted by the maceration method using dichloromethane: Acetone (2:1) for two rounds, each lasting four days. The collected extracts were evaporated and concentrated using a rotary evaporator at 45°C. The concentrated extract (220 g, yield = 4.4%) was dissolved in MeOH: H₂O (70:30) and filtered through a reverse silica gel cartridge made from 10% paraffin-impregnated silica gel to remove highly oily and green contents. The resulting clear and brown filtered solution was concentrated to yield 75 g and then fractionated on a silica gel column (40 - 63 µm; 50 × 450 mm) using a gradient solvent system of hexane: Ethyl acetate, which produced 10 subfractions (90:10; 85:15; 80:20; 75:25; 70:30; 65:35; 0:100).
After checking the TLC profile, which was visualized using 1% cerium sulfate in 5% H₂SO₄, fractions eluted with hexane: Ethyl acetate (85:15, 70:30, and 65:35) that showed pink or light brown spots on the TLC were selected for further analysis. These fractions were submitted to a Sephadex column. Using preliminary 1H-NMR spectra, fractions containing terpenoid resonances were selected and injected into an HPLC column (YMC-Silica, 20 × 250 mm) using hexane: Ethyl acetate (65:35) to obtain compound 1 (4 mg) and compound 2 (20 mg) in their pure state. Compounds 3 and 4 were purified by recrystallization from hexane: Acetone.
3.3. Cell Culture
A2780 cisplatin-resistant (R-CIS) and A2780 Wild ovarian cancer cells, representing R-CIS and non-resistant lines, respectively, were provided by the Mashhad University of Medical Sciences (MUMMS). These cells were cultured in RPMI-1640 media supplemented with FBS, penicillin, and streptomycin (10%, 0.1%, and 0.1%, respectively), and were maintained at 37°C with 5% CO₂ and 95% humidity.
3.4. Cell Viability Assay
The MTT assay for cisplatin and compound 1 was evaluated using the A2780 R-CIS and A2780 wild cells on 96-well plates. A total of 3 × 10³ cells were seeded for 24 hours at 37°C with 5% CO₂. After adding various concentrations of cisplatin (0.1, 1, 5, 10, 50, 100, and 200 µM) or compound 1 (0.1, 1, 10, 20, 40, 100, and 200 µM), the cells were incubated for an additional 48 hours at 37°C in a CO₂ atmosphere. After incubation, 20 µL of MTT reagent (0.5 mg/mL) was added to each well for 4 hours at 37°C, leading to formazan crystal formation. The supernatants were discarded, and the formazan crystals were dissolved in DMSO. The absorbance was read at 570 nm using a Bio-Tek microplate reader (Winooski, VT, USA). Each experiment was conducted in triplicate.
To conduct the combination study in accordance with the study's objectives and the Chou formula (8), it is crucial to maintain a consistent concentration of the tested compound while steadily increasing the concentration of cisplatin. Therefore, we also examined the combination of compound 1 at IC50 with cisplatin at varying concentrations of 0.1, 1, 5, 10, 50, 100, and 200 µM for cell viability assays in both cell lines, as previously mentioned.
3.5. Combination Effect Analysis
For the combination treatment, A2780 R-CIS cell lines were treated in a non-constant ratio with a fixed dose of Malleatin A (IC50) and varying concentrations of cisplatin, or with a fixed dose of cisplatin (IC50) and varying concentrations of Malleatin A for 48 hours. The Combination Index (CI) and Dose Reduction Index (DRI) parameters were calculated according to the Chou equations at the IC50 of the combination therapy (8). Combination Index was calculated using the following equation, with the criteria defined as follows: A CI of less than 1 indicates a synergistic effect, a CI equal to 1 indicates an additive effect, and a CI greater than 1 indicates an antagonistic effect.
The DRI was determined using the following equation, which measures how many times the dose of cisplatin could be reduced in combination with Malleatin A compared to its monotherapy. A DRI greater than 1 is considered highly favorable, especially for avoiding high concentrations of chemical drugs and reducing the risk of adverse effects in conditions such as drug resistance.
3.6. Statistical Analyses
Cytotoxicity values were expressed as the mean ± standard deviation. Statistical analyses were performed using one-way analysis of variance, followed by Dunnett's post hoc test, utilizing GraphPad Prism software.
4. Results
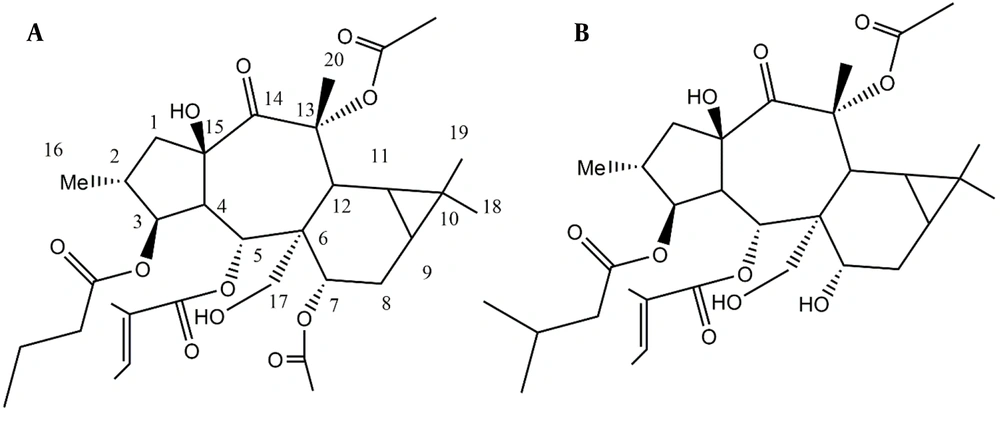
Euphorbia malleata was extracted, and its components were isolated through column chromatography and HPLC, leading to the detection of one steroid, two diterpenes, and one iridoid compound (Figure 1).
4.1. Spectral Data of Isolated Compounds
Compound 1: Colorless oil; for 1H and 13C NMR data, see Table 1; ESI MASS m/z 621.3 (M+H)+.
| Atom | δH (J in Hz) | δC | Atom | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1a | 1.64 m a | 42.99 | 3-OBut-1' | - | 173.07 |
| 1b | 3.17 dd, 13.6, 7.8 | - | 2' | 2.26 m a | 36.23 |
| 2 | 1.86 m a | 37.33 | 3' | 1.56, m a | 18.07 |
| 3 | 5.28, t, J = 3.5 | 78.36 | 4' | 0.93, t, j = 7.7 | 13.88 |
| 4 | 2.34, dd, 11.6, 3.5 | 50.59 | 5-Tig-1' | - | 166.25 |
| 5 | 6.27,d, j = 11.3 | 69.27 | 2' | - | 128.19 |
| 6 | - | 48.09 | 3' | 6.53 d, j = 7.2 | 138.15 |
| 7 | 4.9 m a | 70.6 | 4' | 1.29, d, j = 7.1 | 14.08 |
| 8 | 1.58 m a, 0.98 m a | 22.36 | 5' | 1.47, s | 11.97 |
| 9 | 0.8 m a | 23.98 | 7-OAc-1' | - | 170.17 |
| 10 | - | 18.49 | 2' | 2.16, s | 21.42 |
| 11 | 0.79 m a | 19.08 | 13-OAc-1' | - | 170.74 |
| 12 | 3.55 m a | 35.26 | 2' | 2.13, s | 21.4 |
| 13 | - | 85.74 | |||
| 14 | - | 204.39 | |||
| 15 | - | 84.2 | |||
| 16 | 0.89, d, j = 6.6 | 14.03 | |||
| 17a | 4.56, d, j = 11.7 | 64.01 | |||
| 17b | 4.94, d, j = 11.7 | - | |||
| 18 | 1.75 | 25.01 | |||
| 19 | 0.97, s | 14.97 | |||
| 20 | 1.08, s | 29.54 |
a Overlapping with other signals.
Compound 2: Colorless oil; 1H-NMR data (400 MHz, CDCl3, J in Hz): δH 1.57 (m, H-1a), 3.15 (dd, 13.6, 7.9, H-1b), 1.91 (m, H-2), 5.29 (t, j = 3.44, H-3), 2.42 (dd, 11.04, 3.2, H-4), 6.26 (d, j = 11.3, H-5), 3.90 (m, H-7), 1.34-1.24 (m, H-8), 1.06 (m, H-9), 0.81 (m, H-11), 3.39 (m, H-12), 0.92 (m, H-16), 4.50 (d, j = 11.6, H-17a), 4.92 (d, j = 11.8, H-17b), 1.74 (s, H-18), 1.15 (s, H-19), 1.08 (s, H-20), 2.12 (m, 3-OMB-H-2'a), 2.21 (m, 3-OMB-H-2'b), 1.95 (m, 3-OMB-H-3'), 0.94 (d, j = 7.08, 3-OMB-H-4'), 0.91 (d, j = 7.12, 3-OMB-H-5'), 6.67 (d, j = 7.4, 5-Tig-H-3'), 1.38 (d, j = 6.8, 5-Tig-H-4'), 1.52 (s, 5-Tig-H-5'), 2.13 (s, 13-OAC2'). 13C NMR data (100 MHz, CDCl3): δC 43.39 (C1), 37.83 (C2), 79.05 (C3), 49.8 (C4), 70.93 (C5), 49.08 (C6), 66.43 (C7), 24.56 (C8), 24.28 (C9), 18.92 (C10), 20.11 (C11), 34.54 (C12), 86.49 (C13), 204.69 (C14), 84.22 (C15), 14.63 (C16), 64.6 (C17), 25.67 (C18), 15.56 (C19), 30.07 (C20), 172.94 (3-OMB-C1'), 43.14 (3-OMB-C2'), 25.41 (3-OMB-C3'), 23.07 (3-OMB-C4'), 22.89 (3-OMB-C5'), 166.53 (Tig-C1'), 127.95 (5-Tig-C2'), 140.51 (5-Tig-C3'), 14.63 (5-Tig-C4'), 12.16 (5-Tig-C5'), 171.01 (13-OAC1'), 21.77 (13-OAC2'). ESI MASS m/z 593.33 (M+H)+.
Compound 3: White solid; 1H-NMR data (400 MHz, CDCl3, J in Hz): δH 1.51 (dd, j = 14.4, 3.6, H-2a), 1.95 (t, j = 2.68, H-2b), 4.32 (m, H-3), 1.75 (dd, j = 13.44, 4.04, H-4a), 1.99 (t, j = 2.68, H-4b), 5.68 (s, H-7), 1.44 (s, H-9), 1.25 (s, H-10), 1.76 (s, H-11), 1.34 (s, 3-OH). 13C NMR data (100 MHz, CDCl3): δC 30.65 (C1), 45.53 (C2), 66.70 (C3), 47.19 (C4), 87.01 (C5), 172.28 (C6), 112.79 (C7), 182.85 (C8), 26.45 (C9), 26.96 (C10), 30.65 (C11). ESI MASS m/z: 415.3 (M+H)+.
Compound 4: White solid; 1H-NMR data (400 MHz, CDCl3, J in Hz): δH 3.54 (m, H-3), 5.38 (d, j = 5.2, H-6), 0.69 (s, H-18), 1.03 (s, H-19), 0.95 (d, j = 6.4, H-21), 0.88 (d, j = 7.6, H-24), 0.84 (d, j = 6.8, H-26), 0.84 (d, j = 6.8, H-26), 0.86 (d, j = 6.8, H-27), 0.85 (t, j = 7.4, H-29). 13C NMR data (100 MHz, CDCl3): δC 37.31 (C1), 31.7 (C2), 71.80 (C3), 42.3 (C4), 140.82 (C5), 121.73 (C6), 31.91 (C7), 31.91 (C8), 50.20 (C9), 36.52 (C10), 21.11 (C11), 39.82 (C12), 42.33 (C13), 56.14 (C14), 24.30 (C15), 28.21 (C16), 56.80 (C17), 12.22 (C18), 19.41 (C19), 36.10 (C20), 18.81 (C21), 34.01 (C22), 26.11 (C23), 45.92 (C24), 29.21 (C25), 19.80 (C26), 19.12 (C27), 23.14 (C28), 12.11 (C29). ESI MASS m/z: 197.3 [M + H]+.
4.2. Cell Viability Assay of Malleatin A and Cisplatin
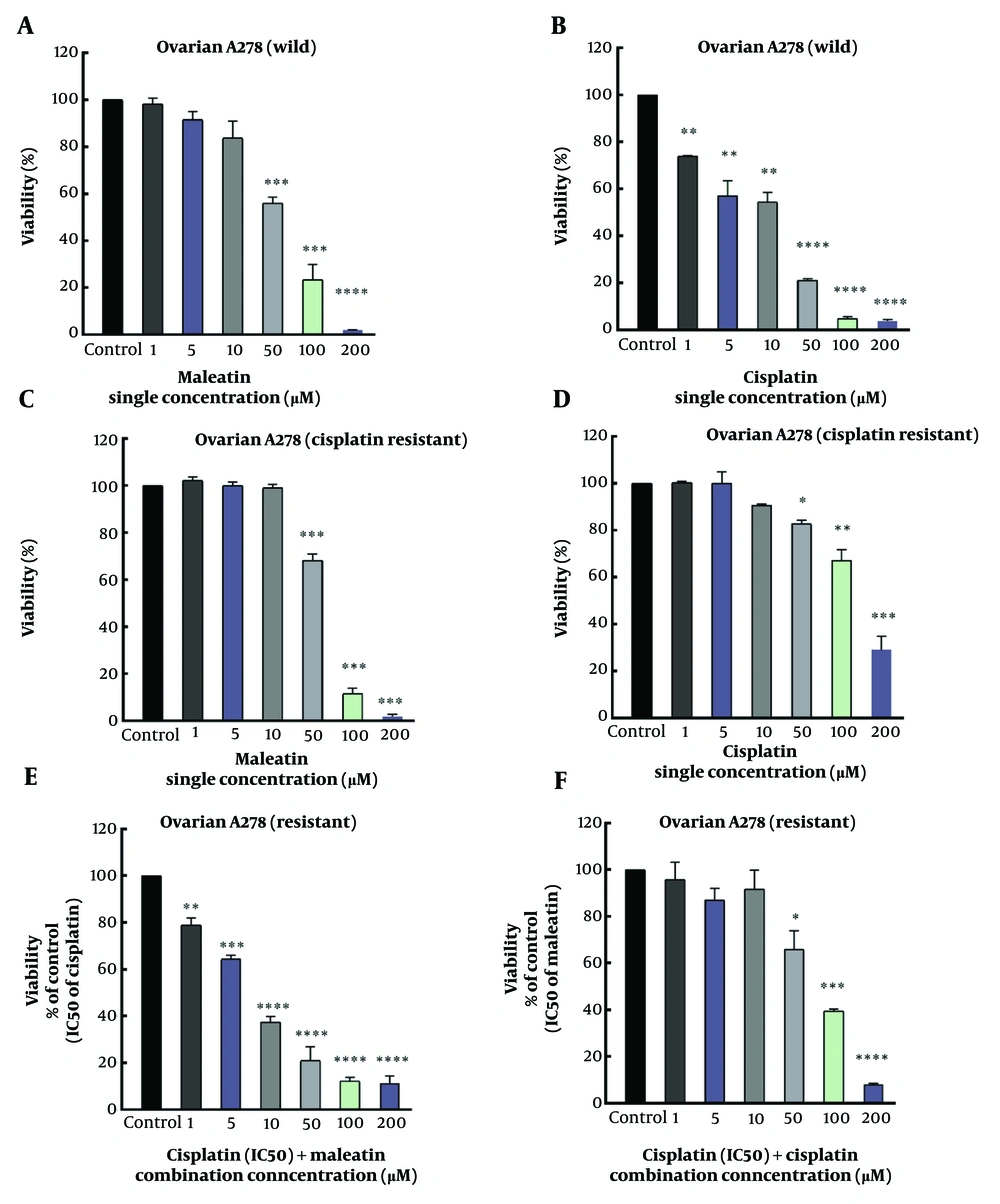
Malleatin A was evaluated against R-CIS and non-resistant ovarian cancer cells to assess its cytotoxicity (Figure 2A and B). Malleatin A demonstrated a cytotoxic effect on the survival of the A2780 wild-type and resistant cell lines, with IC50 values of 56.1 ± 2.74 and 62.5 ± 0.7 μM, respectively. Cisplatin exhibited a cytotoxic effect against the survival of the A2780 wild-type and resistant cell lines, with IC50 values of 12.1 ± 2.2 and 142.5 ± 10.6 μM (Table 2).
Single treatments of Cisplatin (cis) and Malleatin A (mal) against both A2780 wild-type and A2780 cisplatin-resistant (R-CIS) cells, in addition to combination treatments in resistant ovarian cancer cells. (A, B/C, D), MTT assays for A2780 wild-type/A2780 R-CIS single treatments; (E), Combination treatment of a fixed concentration of cisplatin (IC50) while incrementally increasing the concentration of Malleatin A in R-CIS cells; (F), Combination treatment of a fixed concentration of Malleatin A (IC50) while incrementally increasing the concentration of cisplatin in R-CIS cells. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.001.
| Variables and Drugs | Treatment | A2780 Wild Concentration (μM) | A2780 R-CIS Concentration (μM) |
|---|---|---|---|
| Single | |||
| Malleatin A | Alone | 56.1 ± 2.7 | 62.5 ± 0.7 |
| Cisplatin | Alone | 12.1 ± 2.2 | 142.5 ± 10.6 |
| Combination | |||
| Malleatin A | + IC50 of Cisplatin | 18.07 ± 1.5 | |
| Cisplatin | + IC50 of Malleatin A | 39.45 ± 2.9 | |
| CI | 0.58 | ||
| DRI for Cisplatin | 3.65 |
Abbreviations: CI, Combination Index; DRI, Drug Reduction Index.
4.3. Combination Treatment of Malleatin A and Cisplatin
After a 48-hour treatment of A2780 R-CIS cell lines with cisplatin combined with Malleatin A, co-treatment resulted in a significant decrease in cell viability compared to the individual treatments (Figure 2). Figure 2 illustrates that the combination of cisplatin and Malleatin A reduced A2780 R-CIS cell viability more than the single treatments. IC50 values are listed in Table 2.
5. Discussion
Using the standard HPLC method on the YMC-silica column, we separated closely related metabolites (1 and 2). Their NMR resonances were characteristic of polyester and polyol diterpene moieties found in Euphorbia plants.
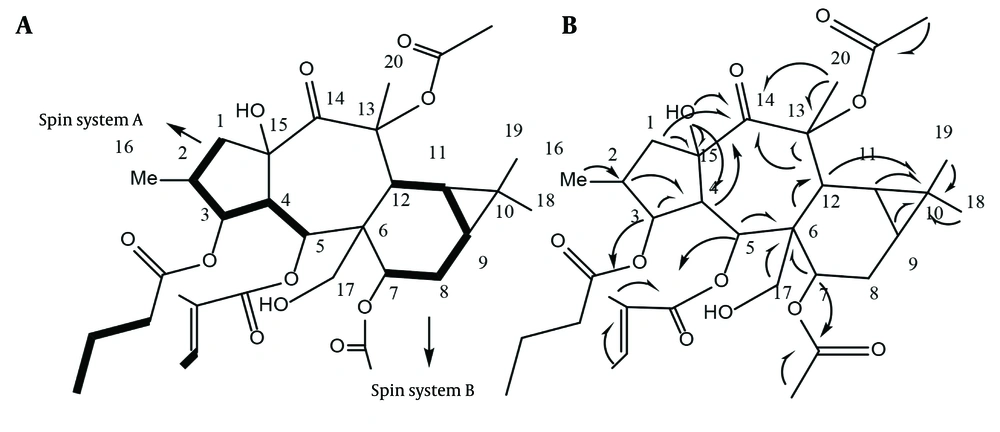
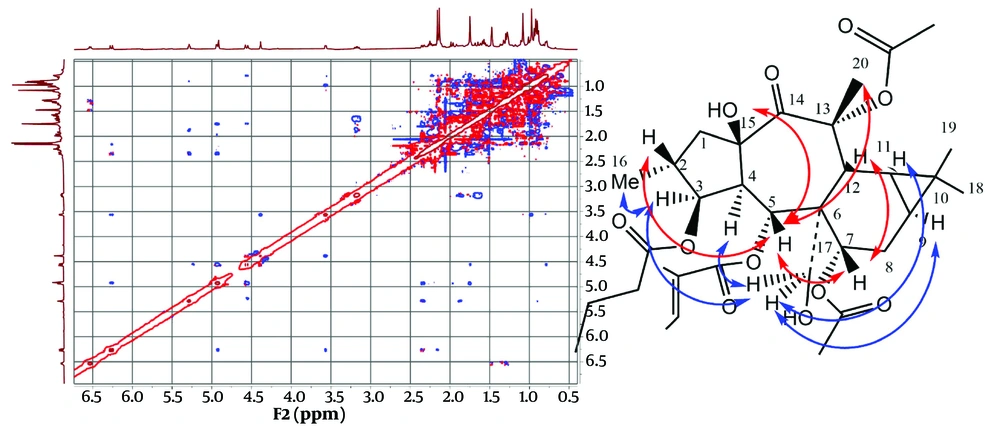
In compound 1, the 13C and 1H spectra revealed a tigliate with chemical shifts at 166.25, 128.19, and 138.15 (δH: 6.53, q, J = 7.2 Hz), 14.08 (δH: 1.29, d, J = 7.1), and 11.97 (δH: 1.47, s), along with a butanoate at 173.07 and 36.23 (δH: 2.26, m), 18.07 (δH: 1.56, m), and 13.88 (δH: 0.93, t, J = 7.7 Hz), as well as two acetate ester groups at 170.17, 21.42 (δH: 2.16, s) and 170.74, 21.40 (δH: 2.13, s) (9). The main structure, devoid of ester groups, contains 20 carbons, including one doublet methyl group at δC: 14.03 (δH: 0.89, d, J = 6.6, Me-16), three singlet methyl groups at δC: 25.01, 14.97, and 29.54 (δH: 1.75, s, Me-18; 0.97, s, Me-19; 1.08, s, Me-20), and three methylene groups at δC: 42.99 (δH: 1.64 m; 3.17 dd, j = 13.6, 7.8, H-1), 22.36 (δH: 1.58 m, 0.98 m, H-8). Additionally, there is one oxygenated carbon at δC: 64.01 (δH: 4.56, d, j = 11.7; 4.94, d, j = 11.7, H-17), eight methines at δC: 37.33 (δH: 1.86 m, H-2), 50.59 (δH: 2.34, dd, j = 11.6, 3.4, H-4), 23.98 (δH: 0.8 m, H-9), 19.08 (δH: 0.79 m, H-11), and 35.26 (δH: 3.55 m, H-12). There are also three oxygenated carbons at δC: 78.36 (δH: 5.28, t, J = 3.5, H-3), 69.27 (δH: 6.27, d, j = 11.3, H-5), and 70.6 (δH: 4.9, overlap, H-7), as well as five quaternary carbons at δC: 48.09 (C6), 18.49 (C10), and two oxycarbons at δC: 78.36 (C13) and 69.27 (C15), along with one ketone group at δC: 204.39 ppm (C14). The DQF-COSY and HSQC-TOCSY spectra assisted in correlating H-1 to H-5 as spin system A and H-7 to H-11 as spin system B in the main structure. Some resonances in the DQF-COSY spectrum overlap; therefore, HSQC-TOCSY was employed to resolve the issue.
Heteronuclear Multiple Bond Correlation (HMBC) was utilized to identify correlations up to three bonds. The HMBC of C6 at δC: 48.09 ppm with H5, H7, and H12 correlated spins A and B through C6. The HMBC of C13 at δC: 85.17 ppm with H-12 and Me-20; C14 at δC: 204.39 ppm with H-12, H-1, and H-4; and C10 at δC: 18.49 ppm with Me-18, 19, and C6 with H-17a,b determined the positions of the quaternary carbons C10, C13, C14, C15, as well as the oxymethylene C17, as shown in Figure 3. The HMBC of δC 173.07 ppm with δH 5.28 ppm; δC 166.25 ppm with δH 6.27 ppm; and δC 170.17 ppm with δH 4.9 ppm located the butanoate function on C3, the tigliate ester on C5, and one acetate group on C7. The remaining acetate group did not show HMBC correlations with any of the oxymethins or oxymethylene and is probably attached to one of the quaternary oxycarbons C13 or C15. The HMBC of C15 at δC 84.2 ppm with the free hydroxy group at δH 4.38 ppm suggested a hydroxy group on C15, with the remaining acetate ester [δC 170.74, 21.40 (δH 2.13, s)] on C13 at δC 85.74 ppm (Figure 3).
Stereochemistry was determined through a ROESY experiment, taking H-4 in the alpha orientation, as indicated by most of the X-ray crystal structures of myrsinane-type diterpenes reported in the literature (10, 11). As shown in Figure 4, the NOE correlations of H-4/H-17 and H-17/H-3 suggested that H-17 and H-3 are in the alpha position. The NOE correlations of H-17/H-9 and H-11 indicated that they have an alpha-cis configuration and, consequently, a triangular ring in the beta orientation. The NOE correlation of H-3/Me-16 determined that Me-16 is in the alpha position, while H-2 is in the beta orientation. The NOE correlations of H-2 (beta)/H-5; H-5/H-7, Me-20; and H-7/H-12 suggested that H-5, H-7, and H-12 are in the beta position. The NOE correlations of H-5 (beta)/15-OH and H-4 (alpha) indicated that the free hydroxy group is in the beta orientation at C15, with the five-membered ring in the trans position fused to the seven-membered rings in the structure. Finally, compound 1 was proposed as 3β-O-butanoyl-7,13α-O-diacetyl-5α-O-tiglioyl-17α,15β-dihydroxy-14-oxopremyrsinane, a newly described compound named Malleatin A (Figure 4).
Compound 2 was isolated as an oil. Its NMR resonances exhibited similarities to those of compound 1. The NMR data revealed the presence of three esters attached to the main polyol structure. The NMR data at δC 166.53, 127.95, and 140.51 (δH: 6.67, q, J = 7.4 Hz), 14.63 (δH: 1.38, s), and 12.16 (δH: 1.52, s) correspond to one tigliate group (9). The NMR data at δC 172.94 and 43.14 (δH: 2.21, m; 2.12, m), 25.41 (δH: 1.95, m), 23.07 (δH: 0.94, d, j = 7.08), and 22.89 (δH: 0.91) are related to a 3-methylbutanoate (12). The NMR data at δC 171.01 and 21.77 (δH: 2.13, s) corresponds to one acetyl ester (9). Excluding the esters, the 13C NMR and DEPT spectra contained 20 resonances, including four methyl groups, three methylene groups (one of which is oxygenated), eight methine groups (three of which are oxygenated), five quaternary carbons, two oxycarbons, and one ketone group. These data resemble the NMR data of the compound 1 backbone but differ by the loss of one 7-O-acetyl group and the presence of a 3-methylbutanoate ester instead of the butanoate group. The loss of the ester group at C7 causes an upfield NMR shift from δC 70.60 (δH: 4.9, m) to δC 66.43 (δH: 3.9, m). The HMBC spectra confirmed the location of the ester groups and determined the structure as 3β-O-3’-methylbutanoyl-13α-O-acetyl-5α-O-tiglioyl-17α,15β-dihydroxy-14-oxopremyrsinane, a newly described compound named Malleatin B (Figure 1). Compounds 3 and 4 were identified as beta-sitosterol and Loliolide, with the C11 iridoid structure, as known compounds consistent with literature data (13, 14).
In the MTT assay, A2780 wild and A2780 R-CIS cell lines were treated with Malleatin A and cisplatin in monotherapy. In the A2780 wild cell line, at lower concentrations of 1, 5, and 10 μM, the cytotoxic activity of Malleatin A was insignificant. However, at higher concentrations of 50, 100, and 200 μM, cytotoxicity was significant, with P-values less than 0.001. Cisplatin exhibited significant cytotoxicity at a lower concentration of 1 μM (P < 0.01). In the A2780 R-CIS cell line, at lower concentrations of 1, 5, and 10 μM, the cytotoxic activity of Malleatin A was not significant, but it became significant at higher concentrations (P < 0.001). The A2780 R-CIS cells demonstrated resistance to cisplatin's cytotoxic activity at lower concentrations of 1, 5, and 10 μM, with no significant reduction in survival. In contrast, at higher concentrations of 50, 100, and 200 μM, cytotoxicity was significant, with P-values less than 0.05, 0.01, and 0.001, respectively, which were significantly lower than those observed in the nonresistant wild type.
Combination therapy was conducted only on the resistant cells. A2780 R-CIS cell lines were treated in two ways: With a fixed concentration of Malleatin A (IC50 value) combined with varying concentrations of cisplatin for 48 hours, or with a fixed concentration of cisplatin (IC50 value) combined with different concentrations of Malleatin A for 48 hours, separately. Co-treatment of A2780 R-CIS cell lines with Malleatin A and cisplatin resulted in a significantly increased mortality rate of the A2780 R-CIS cell lines compared to when cisplatin was used alone. Figure 2 illustrates that the combined treatment of cisplatin and Malleatin A reduced the proliferation of A2780 R-CIS cell lines more effectively than cisplatin alone, lowering their respective IC50 values, as shown in Table 2. The CI and DRI values against the A2780 cells were calculated using the Chou equation (8). For example, in combination treatments of cisplatin with a fixed dose of Malleatin A against A2780 R-CIS cells, the CI was 0.58, indicating synergistic effects.
The DRI measures how much the dose of cisplatin in a combination can be reduced, which is highly important due to its severe side effects. A DRI of 1 indicates no reduction in the drug dose within the combination. A DRI greater than 1 indicates a favorable dose reduction, while a DRI less than 1 suggests an unfavorable dose increase. The DRI value of cisplatin in combination with a fixed dose of Malleatin A against A2780 R- CIS was 3.65, which was favorable (Table 2). It indicates that 3.65 fold the dose of cisplatin is reduced compared to monotreatment in resistant cells.
The cytotoxic effects of Malleatin A were consistent with previous findings regarding the cytotoxic properties of other myrsinane compounds. In a study by Abdolmohammadi et al. (as cited by Mendes et al.), 13(17)-Epoxy-8,10(18)-Myrsinadiene polyester compounds demonstrated cytotoxicity against OVCAR-3 (HTB-161) and Caov-4 (CVCL_0202) ovarian cancer cells, with IC50 values ranging from 35 to 40 µM (15). Proliferin A, featuring a 13(17)-Epoxy-8-myrsinene structure isolated from *E. prolifera*, exhibited cytotoxicity against A2780 wild cells with an IC50 of 7.7 µM (16). Previous research has reported MDR activity in Myrsinane-type compounds, with some myrsinanes displaying MDR activity by inhibiting the rhodamine 123 efflux pump in mouse lymphoma cells (15). Additionally, a study by Vasas et al. explored the synergistic activity of myrsinanes in combination therapy with doxorubicin (17).
5.1. Limitations of the Study
Some fractions with low diterpenoid contents were not determined.
5.2. Conclusions
The aerial flowering parts of E. malleata were analyzed for phytochemicals, resulting in the isolation of two new premyrsinane diterpenes, Malleatin A and B, both featuring a 4-oxopremyrsinane 3β,5α,7,13,17α,15β-hexahydroxy structure. Malleatin A demonstrated cytotoxicity against both A2780 wild and A2780 R-CIS cells, with an IC50 in the range of 50 - 65 μM in the MTT assay. Cisplatin exhibits significant cytotoxicity against the A2780 wild cell line but alone was not sufficient to overcome the resistance exhibited by the A2780 R-CIS cells. However, combination therapy utilizing Malleatin A and cisplatin yields promising results, as evidenced by a synergistic effect that enhances the mortality rate of resistant A2780 R-CIS cells compared to monotherapy. The calculated CI of 0.58 suggests effective synergy, while the DRI of 3.65 indicates a favorable reduction in the required dose of cisplatin, potentially mitigating its side effects. These findings highlight the therapeutic potential of Malleatin A in enhancing the efficacy of cisplatin treatment in resistant ovarian cancer cells, warranting further investigation into combination regimens for improved clinical outcomes.