1. Introduction
Uterine arteriovenous malformations (AVMs) are relatively rare disorders that can cause life-threatening vaginal bleeding. Although angiography is the gold standard of diagnosis, color and spectral Doppler ultrasonography reveal typical images and are useful (1). In the last decade, transarterial embolization (TAE) has become the primary choice for treatment of patients with hemodynamic instability or troublesome hemorrhage, because it can preserve the ability of a successful pregnancy. There are now a handful of published cases of full-term pregnancies after TAE (1-8). This article demonstrates three cases of uterine AVM treated by TAE with their clinical courses, classic images, interventional treatments, and outcomes. The third case in this article had a full-term pregnancy and vaginal delivery without complications.
2. Case Presentation
2.1. Case 1
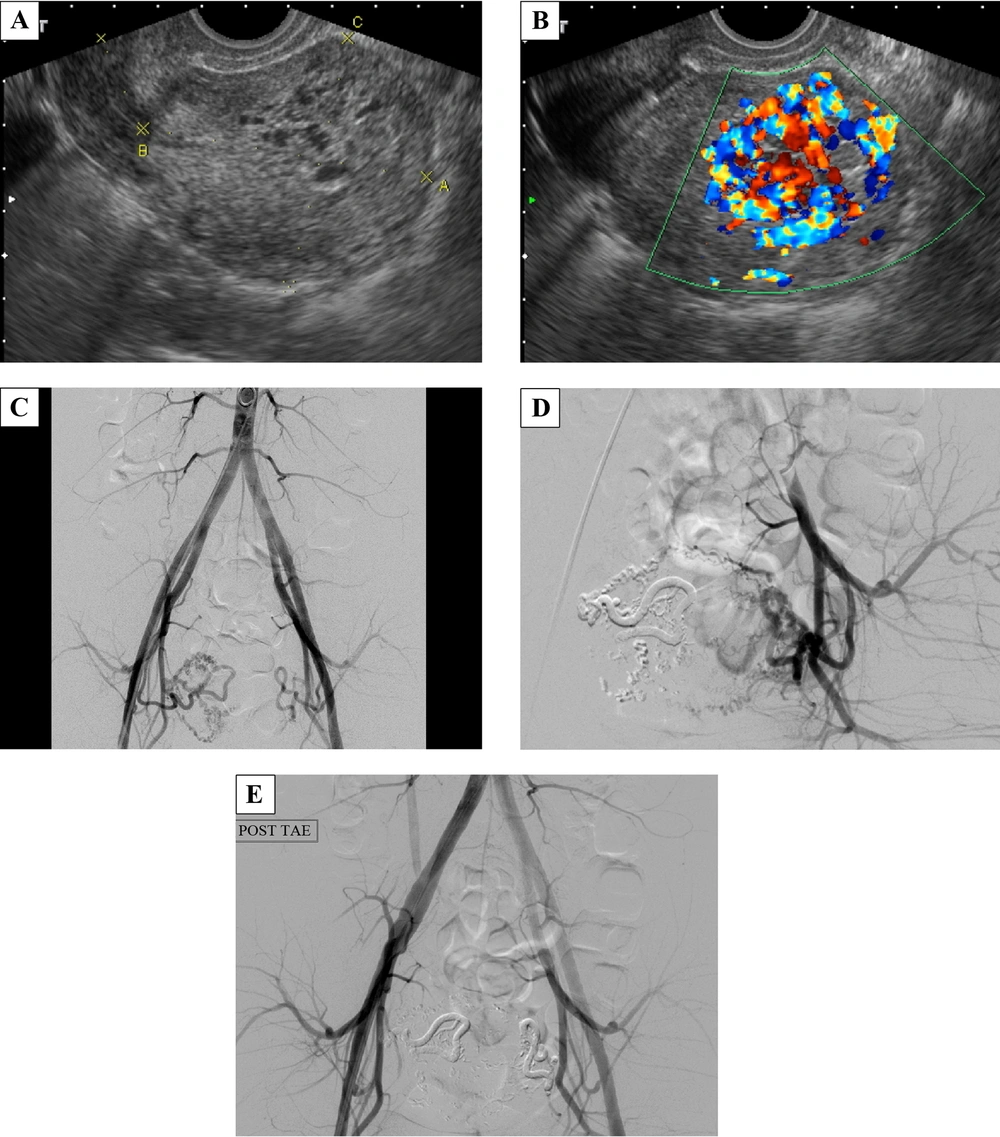
A 25-year-old female, gravida 10, para 0, artificial abortion 10 (G10P0AA10), had a history of dysmenorrhea with irregular duration and intervals of menstrual cycles. She had had no menses for two months before coming to the emergency department with the chief complaint of acute-onset abdominal pain and heavy vaginal bleeding with blood clots. She had neither dizziness nor shortness of breath. Her body temperature (BT) was 37.3°C, heart rate (HR) was 92 times/minute, respiration rate (RR) was 18 times/minute, and blood pressure (BP) was 100/60 mmHg. The pregnancy- enzyme immunoassay test was negative, and beta-HCG was 13.11 mIU/mL, which was in the normal range. In the emergency department, her serum hemoglobin dropped from 13.8 mg/dL to 11.9 mg/dL in 6 hours. The gray-scale ultrasonography revealed multiple varying-sized hypo-echoic lesions (Figure 1A). The color Doppler ultrasonography showed a hypervascular lesion, 4.9 × 3.3 × 2.6 cm with multidirectional turbulent flow (Figure 1B). On admission, the patient was scheduled for angiography and TAE. The angiography showed the feeding artery from the branches of the right internal iliac artery and tortuous veins with relatively early venous return (Figure 1C), which indicated the diagnosis of uterine AVM. Superselective TAE of the right uterine artery using N-butyl cyanoacrylate (NBCA) was performed at first. On checking the left uterine artery, the angiography showed that there were still a few abnormal collateral vessels (Figure 1D). Therefore, bilateral uterine artery embolization was performed (Figure 1E). After the interventional treatment, the patient had mild dull pain in the lower abdomen, which was most likely related to ischemia, and could be relieved by oral non-steroidal anti-inflammatory drugs (NSAIDs). There was no more vaginal bleeding during hospitalization, and she was discharged 2 days after interventional treatment. Forty days after TAE, her menstrual bleeding started, and the follow-up ultrasonography showed no abnormal flow.
A 25-year-old female with a history of dysmenorrhea and irregular duration and intervals of menstrual cycles who came to the emergency department with acute abdominal pain and heavy vaginal bleeding with blood clots. A, Transvaginal gray-scale ultrasonography of the uterus revealed multiple varying-sized hypo-echoic lesions. B, At the same plane, the color Doppler ultrasonography showed a hypervascular lesion with multidirectional turbulent flow (typical mosaic pattern), measuring 4.9 × 3.3 × 2.6 cm in size in the endometrium and myometrium of the uterine fundus. C, During angiography, the catheter was inserted from the right femoral artery and its tip was at the end of the abdominal aorta. Aortography showed the feeding artery from the branches of the right internal iliac artery and tortuous veins with rapid venous shunting. D, After right-uterine-artery embolization, there were still some collateral abnormal vessels formed on the left side. E, Finally, bilateral superselective embolization of the uterine arteries was done smoothly.
2.2. Case 2
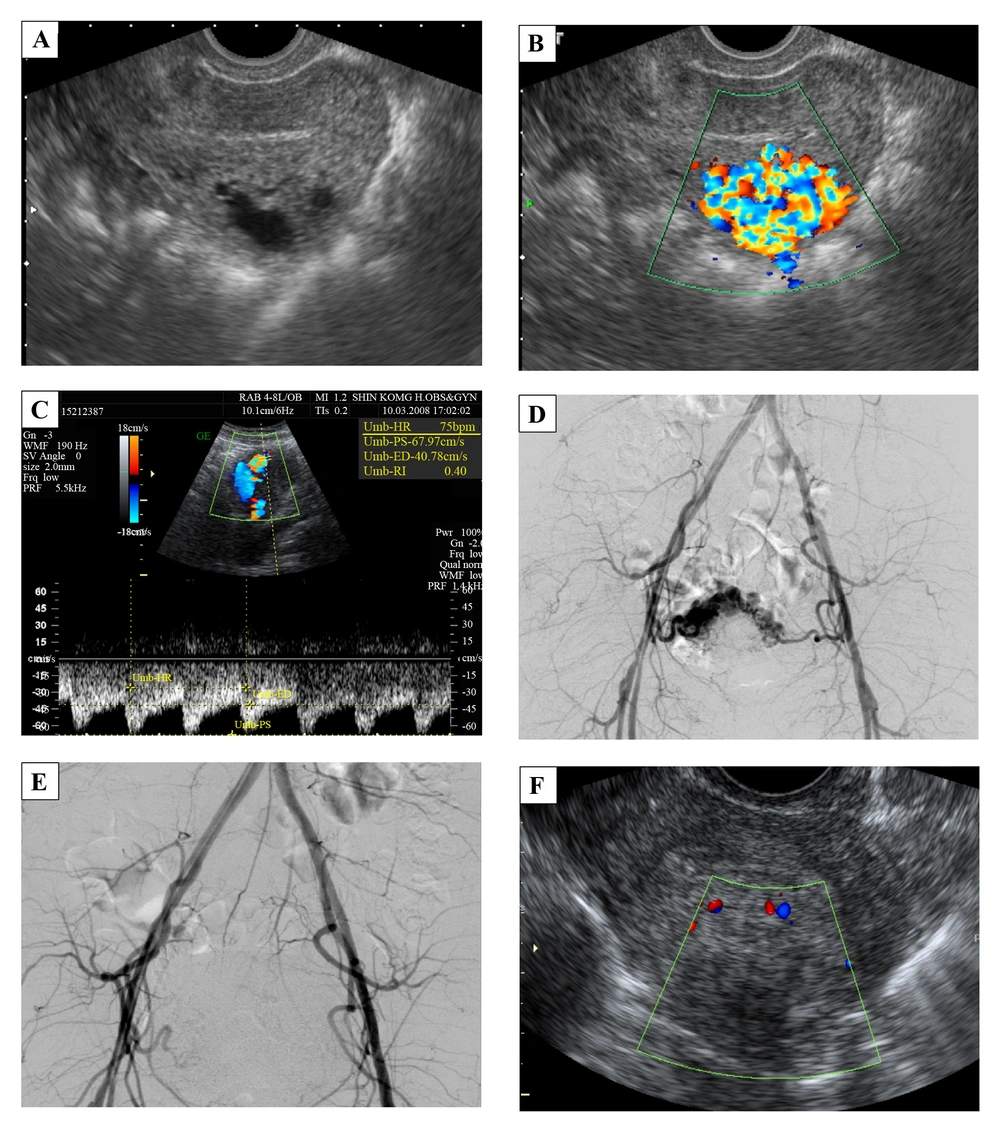
A 38-year-old female, G3P0AA3, with a history of regular 30-day menstrual intervals and 4 - 5 days duration of flow visited our outpatient department with the chief complaint of hypermenorrhea and intermittent severe abdominal pain for 12 days. She was in her usual healthy status until 12 days before when she used a massage machine on her belly to relieve constipation. Then she noted intermittent, severe, and dull lower-abdominal pain accompanied by nausea. The next day the vaginal bleeding started, which she thought was normal menses. Over the following 10 days, she found the pain diffused progressively; there was neither relief nor aggravating factor. The vaginal bleeding was persistent with blood clotting. She had no fever, dysuria, urinary frequency, or purulent vaginal discharge. According to the medical record at outpatient department, her temperature was 36.8°C, heart rate was 84 times/minute, respiratory rate was 20 times/minute, and blood pressure was 120/72 mmHg. The abdomen was soft with tenderness, but there was neither rebounding pain nor muscle guarding. The beta-HCG was in the normal range. The gray-scale ultrasonography revealed hypo-echoic cyst-like lesions (Figure 2A). The color and spectral Doppler ultrasonography demonstrated a hypervascular lesion in the body of the uterus, 2.7 × 2.4 × 2.1 cm with multidirectional turbulent flow, and the resistive index (RI) was 0.4 (Figure 2B and C). The angiography and TAE were arranged afterward. The angiography clearly showed the vascular tangles from the branches of the bilateral internal iliac arteries (Figure 2D). TAE was performed with two microcatheters placed in the bilateral uterine arteries. We injected NBCA slowly till stasis, and the vessels revealed occlusion (Figure 2E). After TAE, the patient had severe abdominal pain and received patient-controlled analgesia for pain control. Because her pain lessened and her condition became stable, the patient was discharged 4 days after treatment. The follow-up color Doppler ultrasonography, which was done 10 days after TAE, confirmed great improvement (Figure 2F). Thirty eight days later, she started normal menstrual periods.
A 38-year-old female with hypermenorrhea and intermittent severe abdominal pain for 12 days. A, Transvaginal gray-scale ultrasonography of the uterus shows hypo-echoic cyst-like lesions. B, The color Doppler ultrasonography demonstrates a hypervascular lesion, 2.7 × 2.4 × 2.1 cm with multidirectional turbulent flow at the body of the uterus. The main lesion is in the myometrium. C, Spectral Doppler ultrasonography shows low resistance index of 0.4. D, Angiography shows vascular tangles from the branches of the bilateral internal iliac arteries. E, Bilateral superselective embolization of the uterine arteries was done smoothly. F, The follow-up color Doppler ultrasonography shows a smaller lesion, 2.0 × 1.3 cm with minimal vascularity.
2.3. Case 3
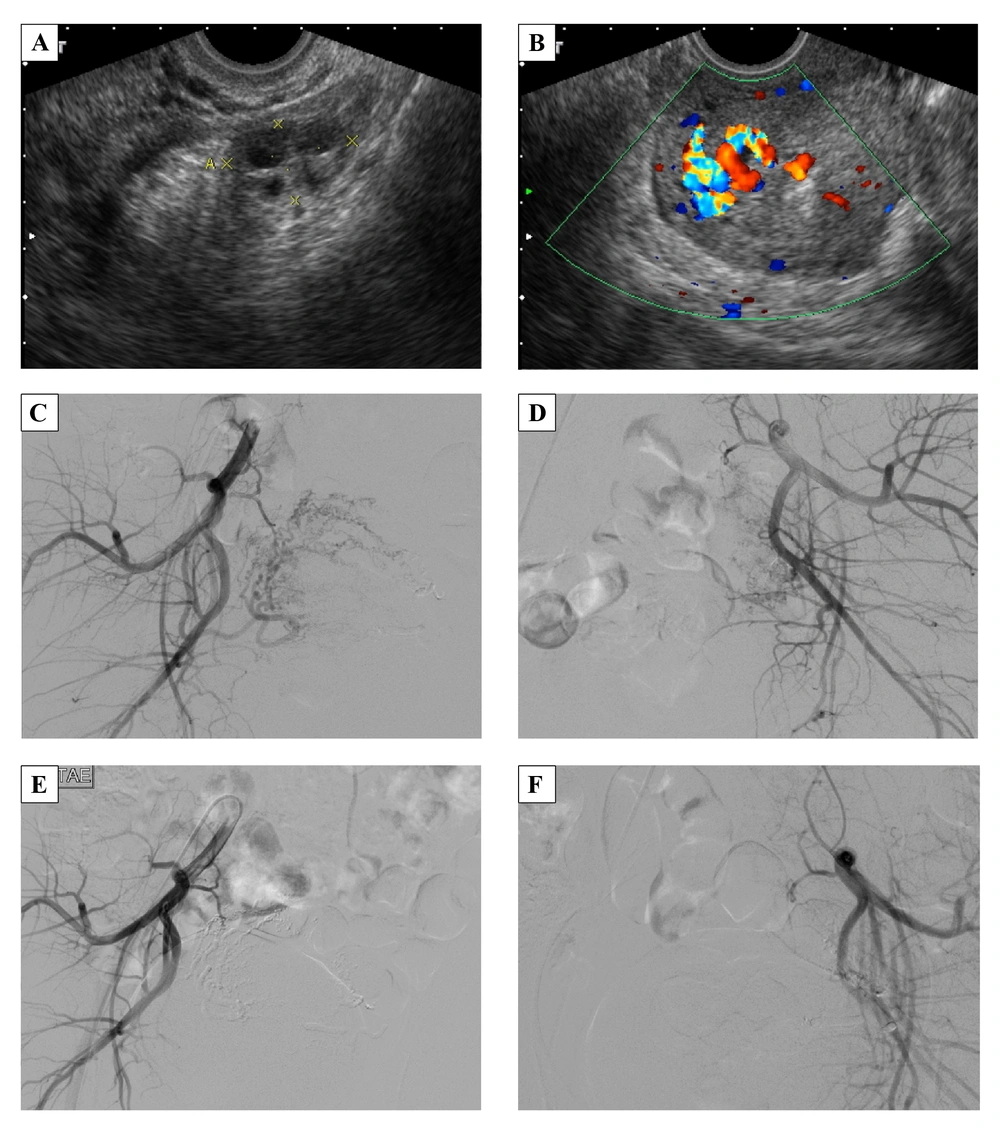
A 22-year-old female, G2P0, spontaneous abortion 1, artificial abortion 1, with regular menstrual cycles in the past had threatened abortion 2 months before she came to outpatient department due to prolonged menstrual flow for 2 weeks with sudden heavy vaginal bleeding. She also noted dizziness and fatigue for days without dyspnea. Her temperature was 36.1°C, heart rate was 76 times/minute, respiratory rate was 18 times/minute, and blood pressure was 116/70 mmHg. The beta-HCG and CA-125 were in the normal range. Gray-scale ultrasonography revealed multiple hypo-echoic myometrial lesions (Figure 3A). Color and spectral Doppler ultrasonography showed a hypervascular lesion, 3.0 × 2.7 × 1.7 cm with multidirectional turbulent flow (Figure 3B), and RI was 0.3. Angiography showed multiple abnormal vessels from the bilateral uterine arteries, particularly from the right side, and rapid venous shunting (Figure 3C and D). TAE was performed using NBCA to obstruct the bilateral uterine arteries (Figure 3E and F). After TAE, she complained of severe lower abdominal pain, which was refractory to demerol. Patient controlled analgesia was used and the pain subsided. She was discharged 3 days after treatment. She had menstruation at 65 days after TAE. Three years later, she was admitted to our hospital for normal spontaneous delivery with a gestational age of 39 weeks. The baby girl was healthy, Apgar scores were 9/9, and body weight was 3020 gm. The placenta was delivered by complicated manual removal with both surface (Duncan and Schultze), but there was no gross abnormal finding, including cord abnormalities. Her prenatal examination was uneventful, and there were no complications during delivery or the postpartum period.
A 22-year-old female with prolonged menstrual bleeding for 2 weeks and sudden heavy vaginal bleeding. A, Gray-scale ultrasonography reveals multiple hypo-echoic myometrial lesions. B, Color Doppler ultrasonography shows a hypervascular lesion, 3.0 × 2.7 × 1.7 cm in the endometrium and myometrium of the uterine fundus. C and D, Angiography of the right and left uterine arteries demonstrates multiple abnormal vessels from the bilateral uterine arteries, particularly from the right side, and rapid venous shunting. E and F, After treatment, angiography shows total occlusion of AVM.
3. Discussion
Uterine AVM is a relatively rare disorder that can cause life-threatening bleeding. They are still limited to a few case reports, a small number of series, and fewer review articles. Even though there is no large-sample analysis, O’Brien et al. report the rough incidence as 4.5% by performing ultrasonography in 464 pelvic-bleeding patients, ages 18 to 41 years (1). Uterine AVMs may be congenital or acquired, the latter being more common. Acquired uterine AVMs may be caused by tissue damage and are followed by abnormal vascularization during the healing process. Most patients have a history of pregnancy (with delivery, spontaneous miscarriage, or surgical abortion) or dilation and curettage (D & C) (2, 9). Each of the three cases we described had a history of multiple abortions. Uterine AVMs can also be associated with infection, trophoblastic disease, malignancy, and exposure to diethylstilbestrol (2, 9). Hormonal change, such as pregnancy, menstruation, high-dose continuous estrogen and progestin therapy also play an important role in uterine AVMs because they may induce a bleeding episode, and most cases are in the range of 20 to 40 years of age (6).
The common clinical manifestations include abnormal vaginal bleeding (from spotting to sudden heavy bleeding) and lower abdominal pain. Throbbing discomfort in the lower abdomen, urinary frequency or incontinence, dyspareunia, hypotension, and cardiac failure have also been described (2). The patients in these three cases all have the common clinical presentation with a negative beta-HCG. The second patient also had a history of applying external force to her belly with a massage machine, which may have triggered her symptoms. There is no specific physical finding or sign, but pulsatile lesion, although rare, has been reported by manual pelvic examination.
Ultrasonography is a convenient imaging modality for evaluation of patients with vaginal bleeding; however, gray-scale ultrasonography does not provide powerful evidence to differentiate in this situation (1). The color and spectral Doppler ultrasonography give us much more information to accurately approach the diagnosis of uterine AVMs. Classically, the color Doppler ultrasonography demonstrates the vessel structure with multidirectional high-velocity flow that produces a “color mosaic pattern,” and the spectral Doppler analysis typically shows high-velocity flow with a low resistance index that indicates arteriovenous shunting (1). Nowadays, angiography is the gold standard of diagnosis. Nevertheless, it may not be used in every case unless physicians plan to do the following TAE or to use it for study purposes. Angiography of uterine AVMs shows, as is the case with AVMs at other sites, tortuous abnormal vessels between feeding arteries and early-contrast-enhancing drainage veins.
A wide spectrum of management plans have been proposed for uterine AVMs including observation, oral medications, TAE, laparoscopic bipolar coagulation, surgical ligation of uterine arteries, and hysterectomy (7, 10, 11). The management of choice is decided according to hemodynamic stability or the need to control acute, heavy hemorrhage. Patients with asymptomatic or mild symptomatic uterine AVMs need no initial treatment but only follow-up ultrasonography, and the condition usually resolves spontaneously within months (9). Oral contraceptive medications may also have a role in this population. On the other hand, hysterectomy is the traditional choice for stopping the troublesome bleeding of uterine AVMs. In the last decade, more and more published case reports have chosen to treat by TAE, and the clinical success rate is greater than 90% in retrospective review articles (12, 13).
Compared with hysterectomy, TAE can preserve reproductive ability ideally, but physicians must be concerned about the embolization resulting in decreased blood supply to the uterine and whether it may lead to failed pregnancy. If the embolic materials flow into the ovarian artery, ovarian function and fertility may be disturbed. There are a lot of studies which discuss ovarian function and pregnancy following arterial embolization for postpartum hemorrhage (PPH) or uterine fibroids, but the issue is still controversial. Most articles suggest that uterine artery embolization does not cause higher risks of impairment of ovarian function (14), fertility (15), or major pregnancy outcome. (14-18). However, adverse effects, including synechia (19), recurrent PPH (19), miscarriage (20), and loss of ovarian reserve (21) are reported. The last review article suggests that uterine TAE does not have an observable effect on the ovary in most women younger than age of 45 years (8). Berkane et al. pointed out that the smaller the embolic particles are, the higher risks of occlusion of the ovarian arteries will be, and they mention that the probable cut-off for material size is 500 μm (19). Many case reports have transient impairment of ovarian function after embolization. They have normal menstrual cycles weeks to months later.
Currently, there is no strong evidence about TAE induced persistent ovarian failure, major complications of pregnancy, or fetal growth restriction. Case reports in this article and a handful of other documented reports describe full-term pregnancy after TAE. Searching on PUBMED, there are 21 cases that have term pregnancy after TAE for uterine AVMs (1-8), including one case with twin babies. The three cases in this article received TAE treatment with NBCA to control acute heavy bleeding and to protect the patients’ desire for a successful pregnancy. Varying severity of lower abdominal pain associated with post-TAE ischemia is noted in these patients and is also common in the past reports. Mild pain can be relieved by just oral NSAIDs; severe pain can be controlled well with patient-controlled analgesia use and will subside in a few days. The three cases were followed up at our gynecologic outpatient department for 2, 3, and 6 months, and there were no recurrent AVMs. Case 3 had a full-term delivery, with a gestational age of 39 weeks, three years after TAE without complications during pregnancy, delivery, or the postpartum period. The placenta had no gross abnormality.
In conclusion, TAE is a safe and effective treatment for uterine AVMs when the patient has hemodynamic instability or refractory hemorrhaging. If the patient wants to preserve her reproductive ability, TAE is the first management of choice. Our report enhances the confidence in achieving full-term pregnancy and vaginal-route delivery after superselective TAE in patients with uterine AVMs. In the future, more studies with high quality of evidence for side-effects after TAE and statistical analysis of risk factors are needed.