1. Introduction
Dermatofibrosarcoma protuberans (DFSP) is a mesenchymal tumor of the dermis and subcutaneous tissues (1). It is a slow growing low- to intermediate-grade malignancy with a high propensity for local recurrence, especially if negative resection margins are not achieved. However, metastases are rare (2).
DFSP most commonly involves the trunk, followed by extremities, head and neck. Because occurrence in the breast is very rare, DFSP is often misdiagnosed as a benign breast tumor, leads to treatment delay. Only a few single case reports with imaging findings have been published (2). We reported a patient with DFSP of breast together with characteristic imaging features of various radiologic modalities including mammography, ultrasound, MRI and fludeoxyglucose-positron emission tomography computerized tomography (FDG-PET CT), which are necessary for an accurate diagnosis and providing a differential diagnosis from other breast lesions.
2. Case Presentation
A 41-year-old female presented with a lump in her left breast that she had noticed it about one month previously. Physical examination revealed a firm, tender nodule at 12-o’clock position of the left breast. No regional skin color changes were observed. She had no specific clinical history or family history of breast cancer.
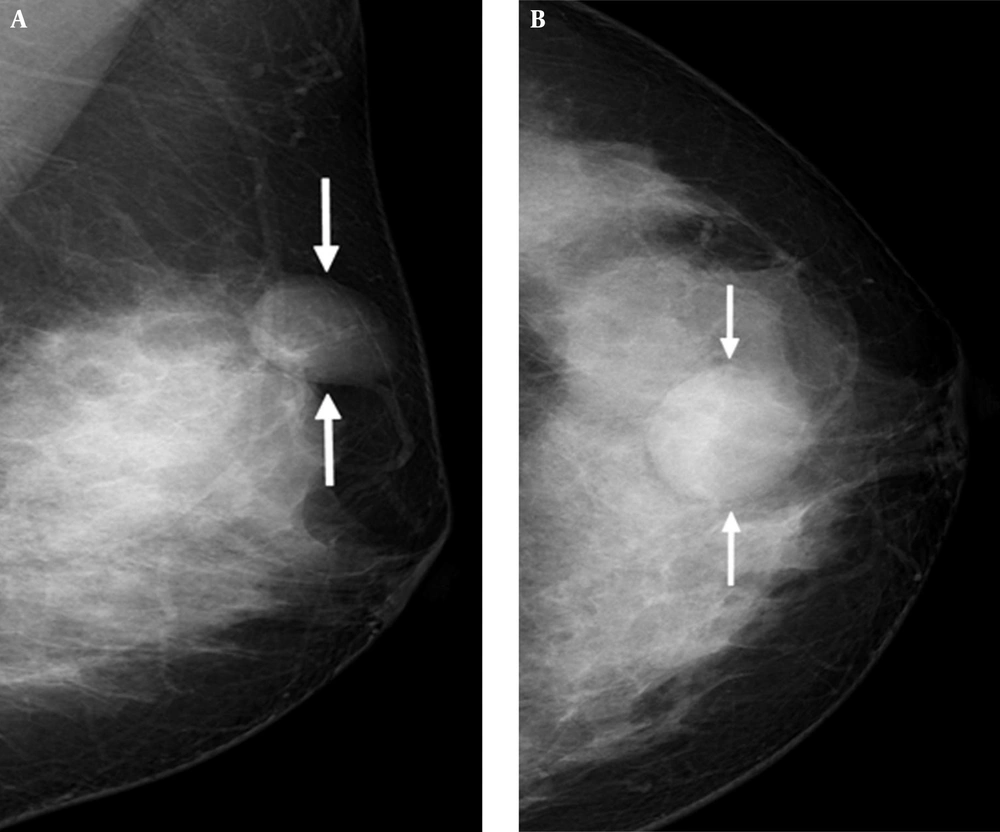
Mammography showed heterogeneously dense breasts that would be categorized as breast imaging reporting and data system (BI-RADS) type C. It also revealed a circumscribed, oval-shaped, equal-density mass in the subcutaneous fat layer of the upper central portion of the left breast. Associated abnormal calcification or architectural distortion was not observed (Figure 1A and B).
A 41-year-old female presented with a lump in her left breast. Mediolateral oblique view (A) and craniocaudal view (B) of mammography shows dense breast and a circumscribed, oval-shaped, equal-density mass (arrows) in the subcutaneous fat layer of the upper central portion of the left breast.
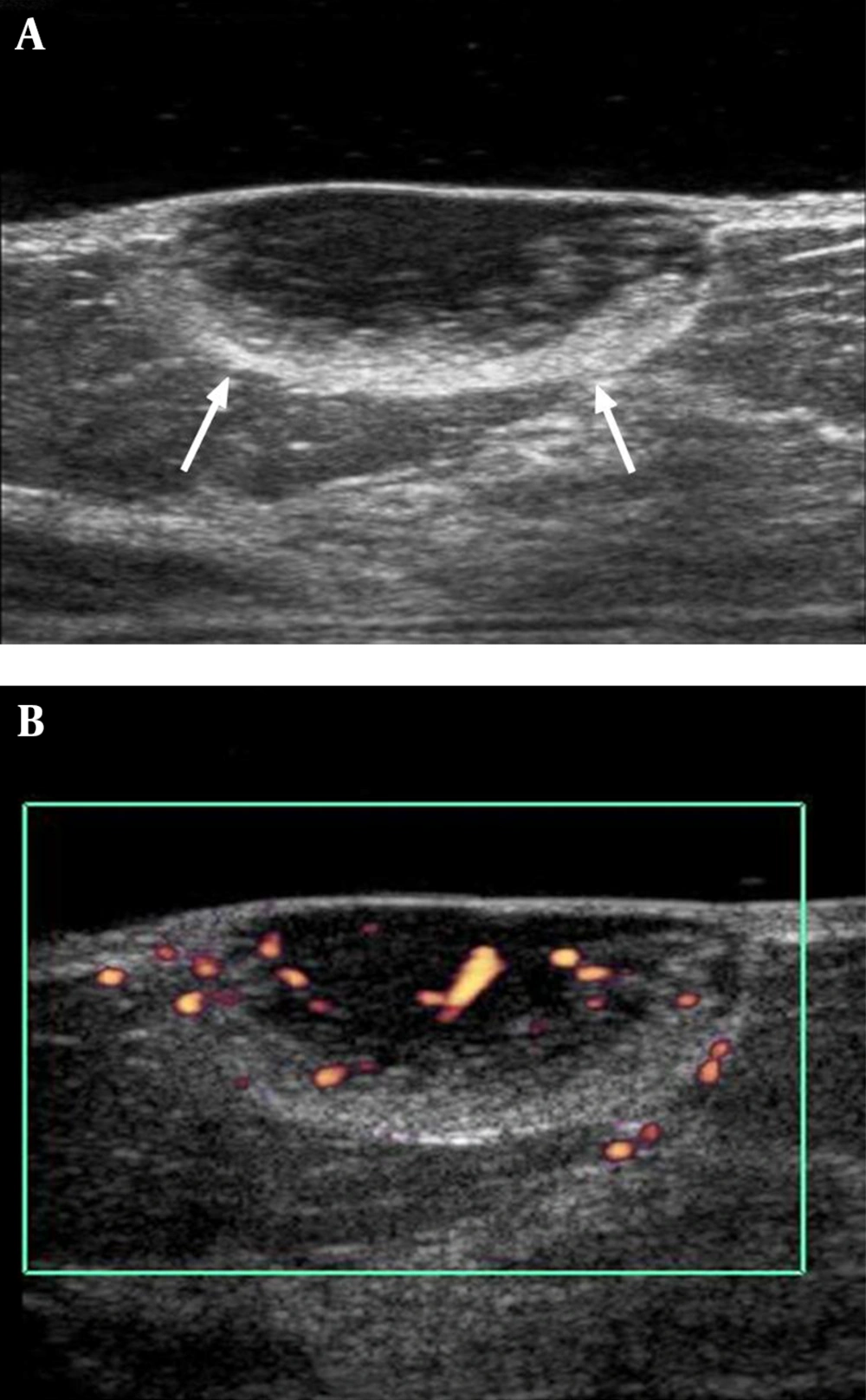
Subsequent ultrasonography performed using an acoustic gel pad demonstrated a 2.4 × 1.0 cm, circumscribed, oval-shaped mass in the skin and subcutaneous fat layer at the 12-o’clock position of the left breast, 4 cm from the nipple. The mass had a heterogeneous echo pattern with a peripheral hyperechoic rim (Figure 2A) and increased vascularity on Color Doppler sonography (Figure 2B). Malignancy was suspected for this lesion and it was categorized as BI-RADS 4. Ultrasonography-guided core biopsy was performed and spindle cell neoplasm was considered.
A, Gray scale image of ultrasonography showing a 2.4 × 1.0 cm circumscribed, oval-shaped, heterogeneous echoic mass with peripheral hyperechoic rim portion (arrows) in the subcutaneous fat layer at the 12-o’clock position of the left breast, 4cm from the nipple; B, Color Doppler image of ultrasonography showing increased vascularity in mass.
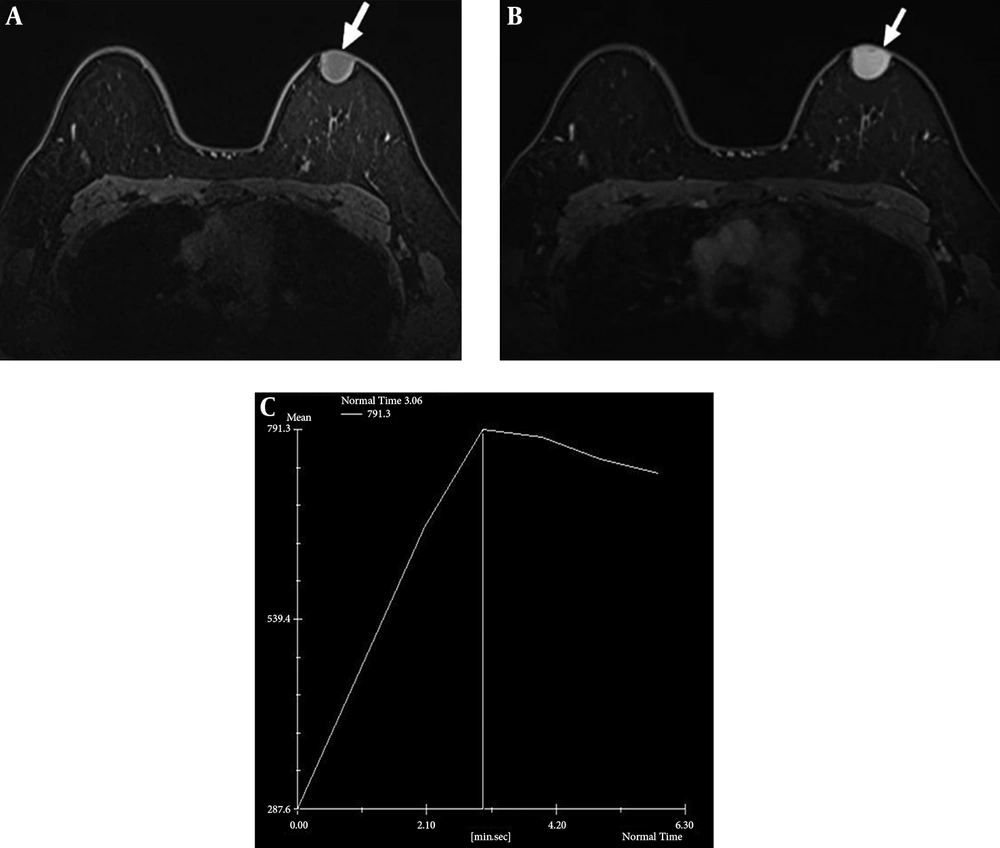
Dynamic contrast-enhanced breast-magnetic resonance imaging (MRI) was performed using a 1.5-T system with a dedicated breast coil, which revealed a 2.4 × 2.0 cm circumscribed oval mass with a wide base to the skin. The mass was observed as low signal intensity on T1-weighted images with homogeneous strong enhancement (Figure 3A and B). Most sections of the tumor showed rapid initial enhancement with washout kinetics (Figure 3C).
Axial T1-weighted magnetic resonance image shows circumscribed oval low signal intensity mass in the left breast (A, arrow) and homogeneous strong enhancement in majority of mass could be seen after contrast injection (B, arrow). Kinetic curve shows early strong enhancement with washout (C).
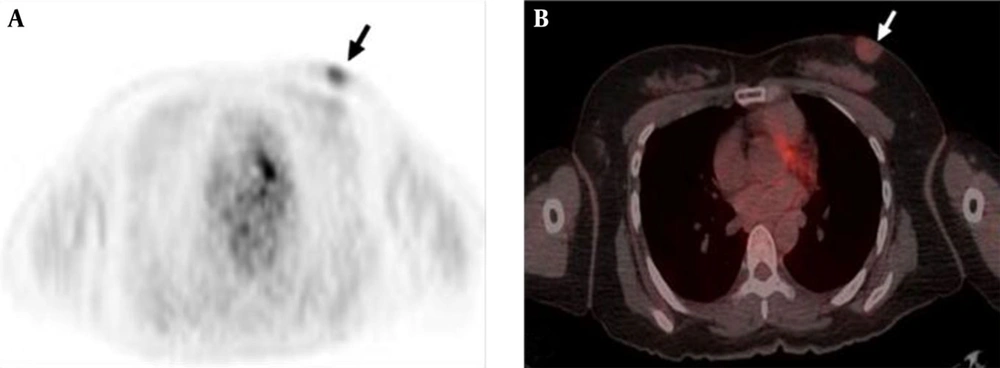
PET-CT was performed for a routine evaluation of distant metastasis. 18F-FDG-PET demonstrated faint FDG uptake around the mass in the left breast and standardized uptake value (SUV) was measured as 1.8 (Figure 4A and B). Other soft tissue FDG uptake or evidence of distant metastasis was not observed.
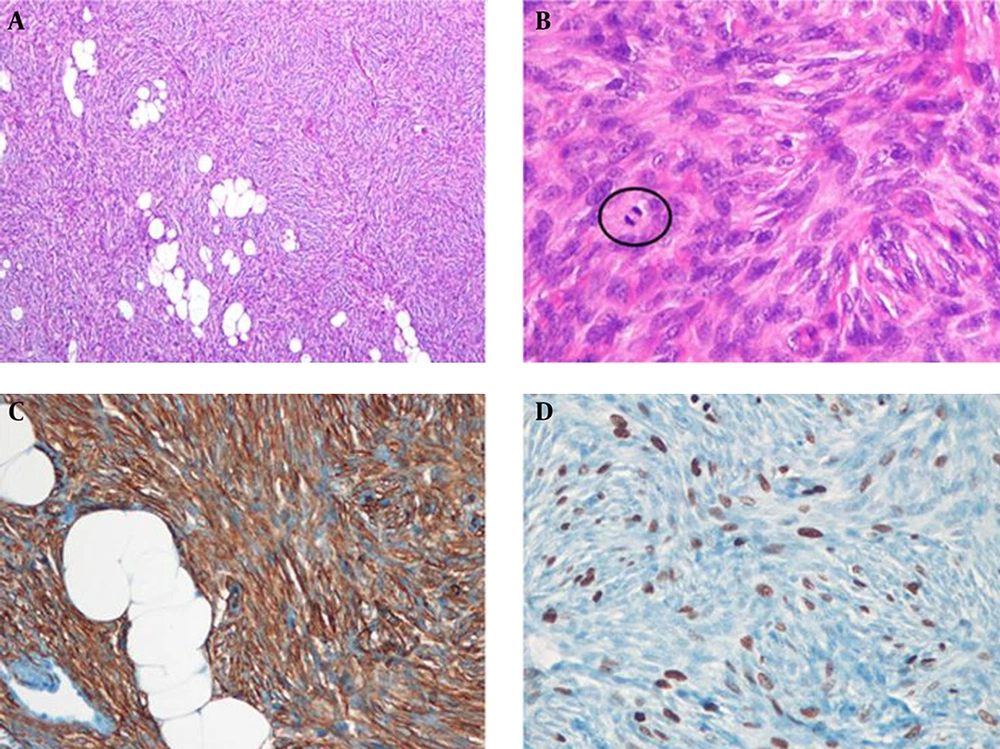
The patient underwent local mass excision with 3 cm margins. Histologic examination revealed infiltration of a 2.0 × 1.5 × 2.0 cm sized tumor into the subcutaneous fat on low-power field view and storiform arrangement of short spindle cells on high-power field view. The tumor cells were diffusely positive for CD34 immunostaining (Figure 5). Therefore, histologic diagnosis was DFSP. Tumor recurrence was not observed during sonographic follow-up 4 years after surgery.
Histopathologic findings of the left breast mass. A, At low magnification view, the tumor is homogeneously cellular and infiltrates into subcutaneous fat (Hematoxylin and eosin (H & E) stain, × 40); B, High power view demonstrates storiform arrangement of short spindle cells and mitotic figures (circle) (H & E stain, × 400); C, Tumor cells have diffuse positive results for CD34 immunostaining (× 200); D, Ki-67 labeling index is 25% (× 200).
3. Discussion
DFSP is a rare cutaneous malignancy that arises from dermis and subcutaneous fat layer. The reported incidence is around 5 cases per 1 million persons annually and is most commonly found in adults aged 20 and 50 years (1). Although the tumor is a low-to-intermediate grade sarcoma, it can infiltrate into the surrounding deeper tissue with a propensity for local recurrence, especially after inadequate excision. While metastasis is rare, it can occur in distant sites, especially the lung (2-4). Furthermore, differentiation into higher-grade sarcomas with higher metastatic potential tends to occur in poorly treated recurrent tumors (1).
DFSP most commonly appears on the trunk (50 - 60%), extremities (25%) and in the head and neck (10 - 15%). Although breast involvement is quite unusual, it has been reported in the literature with imaging features (1).
The reported mammographic findings of DFSP are nonspecific, i.e. a skin-based or intramammary-located, oval, circumscribed mass without calcification that mimics a benign lesion (1, 2, 5). These features are consistent with our case, in which a circumscribed, equal- density, oval mass without calcification was observed in superficially subcutaneous fat layer of the upper central portion of the left breast.
The characteristic ultrasonographic features of DFSP of breast were a circumscribed, hypoechoic, subcutaneous mass with increased internal blood flow in Color Doppler image (4, 6, 7). Especially, hypervascularity of the tumor helps it to be differentiated from other benign breast lesions. Although a breast abscess or complicated epidermal inclusion cyst such as infection or rupture can appear as a hypoechoic mass with a thick echogenic periphery and increased vascular flow (8). However, our patient had no clinical symptoms associated with infection such as pain, redness, a sense of heating or fever. Another distinct ultrasonographic feature of DFSP of the breast is a peripheral hyperechoic band like rim in the subcutaneous tissue, which represents a mixture of tumor cells and fibrous tissue infiltrating the subcutaneous fat (2).
Although the signal intensity of DFSP on breast MR imaging is nonspecific, MRI is the most helpful imaging modality for evaluating the tumor extent and anatomical relationships with surrounding structures. Compared to ultrasound, MRI represents more accurately the tumor size and the boundary because of excellent contrast with surrounding tissue. MR appearance of breast DFSP is a well-circumscribed, intermediate low-intensity tumor on both T1 and T2-weighted images with washout-type kinetics and strong enhancement (4). We also observed a low signal intensity mass on T1-weighted images with initially rapid strong enhancement with washout kinetics in our case.
Although DFSP has a high tendency for local recurrence after insufficient surgical excision, metastasis is rare. However, distant metastasis may occur at the lung, bone or regional lymph nodes after repeated local recurrence (9, 10). FDG-PET is a useful tool for predicting tumor recurrence and distant metastasis, detecting multicentric tumor origin and providing relevant anatomical information (11). A few reports have described PET-CT findings of DFSP including mild uptake in the skin of the trunk and head or more marked uptake at metastatic sites in the lung, retroperitoneum or muscles (12, 13).
Folpe et al. (14) reported a highly significant relationship between the highest PET SUV values and cellularity and proliferative indices in sarcomas. In the present case, the tumor showed mild FDG uptake with a SUVmax of 1.8. Although a correlation between FDG uptake and histologic grade in DFSP has not been reported in the literature, we assume that low SUV of the DFSP of the breast was attributable to low cellularity and proliferative indices. To our knowledge, this is the first case showing 18F-FDG PET findings of breast DFSP.
In conclusion, breast DFSP is a rare sarcoma that may simulate a primary breast mass both clinically and radiographically. We described a case of breast DFSP and presented radiologic features that can potentially help provide a differential diagnosis between DFSP and primary benign breast lesions. Mammography, ultrasound, MRI and FDG-PET findings of a subcutaneously located, highly vascular, non-inflammatory tumor with a wide base to the skin might be suggestive of DFSP.