1. Background
Ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles have been used as a blood pool (intravascular) contrast agent. They are useful for magnetic resonance angiography (MRA), assessing myocardial and renal perfusion, and myocardial injury (1-3). Intravascular contrast agents have higher relaxivity than the extracellular agents (4). Chambon et al. reported that T1 relaxivity of iron oxide nanoparticles is 5 to 6 times higher than the gadolinium chelates (5). The USPIO nanoparticles also have long blood half-life and provide adequate time for data acquisition in MR imaging. Therefore, intravascular signal intensity (SI) loss is little (6). SI of MR images depends on the magnetic field strength (7), size (8) and coating type (9) of the nanoparticles, concentration of the contrast agent such as gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA) (10, 11) or iron oxide nanoparticles (9), and imaging pulse sequences (e.g. spin echo, fast spin echo, and inversion recovery) and parameters (e.g. repetition time (TR), echo time (TE), inversion time (TI), and saturation time (TS) (12-18).
Previous studies using iron oxide nanoparticles showed that the flip angle exerts an important effect on the SI of the MR images and contrast of MRA (4). Different studies were performed to evaluate the flip angle effect on the MRA image quality (19) and to determine appropriate flip angle for MRA using iron oxide nanoparticles and different MRI pulse sequences (20, 21). They reported different flip angle values as the appropriate flip angle that leads to highest MR signal intensity for MRA. Anzai et al. reported little flip angle dependence on the image quality for abdominal MRA using USPIO nanoparticles (19). A study carried out by Mayo-Smith et al. showed that the maximum SI in blood samples occurred at flip angles of 30º and 60º before and after administration of AMI-227, respectively (20). Tanimoto et al. found the optimal flip angle of 20º for the inferior vena cava before and after USPIO injection (21).
Although evaluation of flip angle effect on SI and determination of appropriate flip angle has been investigated for MRA, in current MR perfusion studies, optimum flip angle has not been determined. For accurate perfusion measurement, it is important to find a flip angle that leads to the maximum linearity at the highest concentration to improve signal to noise ratio (SNR). The investigators still use different flip angles and pulse sequences for their perfusion measurement studies. However, it has not been cleared yet that which flip angle is optimum when we use the inversion recovery pulse sequence with an inversion time (TI) that suppresses the blood signal for in vitro situation.
2. Objectives
The aim of this study was to investigate the effect of flip angle on the linear relationship between SI and different concentrations of iron oxide nanoparticles in T1-weighted MR images using inversion recovery pulse sequence to find the optimum flip angle which is suitable for perfusion measurement. In addition, the maximum SI at different concentrations and different flip angles was also investigated using USPIO nanoparticles with carboxydextran coating.
3. Materials and Methods
3.1. Theory
The relationship between SI and imaging parameters in the inversion recovery sequence can be written as (12):

Where S (t) is the SI after administration of the contrast agent, and S0 is the observed SI when there is no contrast agent. T1, TE, and T2 denote the tissue longitudinal relaxation time, the echo time and transverse relaxation time, respectively (15).
The relationship between SI and concentration of contrast agent is described by the following equation:

Where T1Pre is the longitudinal relaxation time before contrast application, C(t) is the concentration of the contrast agent at time t, and K is a constant that depends on the contrast medium (15).
Equation 2 with respect to the effect of flip angle on SI can be written as (22):

Where α is flip angle. It is necessary to mention that exp(-TET2) can be ignored from equations 1, 2, and 3 at lower concentrations of contrast agents. The T1-shortening effect is dominant at low concentrations. Therefore, the relationship between SI and concentration is linear. At high concentrations, both T1 and T2 can be affected as SI response becomes nonlinear with the concentration (14, 15).
3.2. Contrast Agent
Nanomag-D-spio nanoparticles (Micromod Partikeltechnologie GmbH, Rostock, Germany) with a core size of 7 nm, mean diameter of 20 nm and carboxydextran coating were used for this study. The concentration of iron in the ferro-fluid was measured as 1.29 mg/mL using atomic absorption spectrometer (analytik jena, novAA® 400, Germany). Then 1.8 mL of the ferro-fluid was diluted using 48.2 mL deionized water to prepare 50 mL of the highest concentration (500 µmol Fe/L). Other concentrations were prepared with dilution of this sample with appropriate volumes of deionized water. Concentration of the nanoparticles varied between 0 and 500 µmol Fe/L (0-100 µmol Fe/L with interval of 5 µmol Fe/L and 100-500 µmol Fe/L with interval of 100 µmol Fe/L).
3.3. Phantom
A Perspex phantom was designed with dimensions of 13 × 13 × 13 cm to placement of glass via ls (internal diameter = 15 mm) filled with various concentrations or constant concentrations of iron oxide nanoparticles (Figure 1). The phantom consisted of 25 vials with different and constant concentrations of nanoparticles that were carefully placed in the center of the standard clinical MRI head coil. Figure 1 shows a coronal image of the phantom with different concentrations.
Non-uniformity of the coil is a major challenge in MRI. To measure the accurate SI of an MR image, the response of the RF coil should be uniform. Therefore, 25 vials with constant concentration of iron oxide nanoparticles (50 µmol Fe/L) were placed in exactly the same positions of the vials with different concentrations to measure the coil non-uniformity. The SI of one vial in the center of the coil was chosen as reference SI (e.g., the vial with concentration of 60 µmol Fe/L). The SI of all vials with the constant concentration should be equal to that SI. After normalization of the mean SI from the vials with constant concentration, the correction factor due to non-uniformity of the coil was calculated for different positions of the vials with different concentrations. To calculate the corrected SI for different concentrations, the SI of each vial was multiplied by its correction factor (15, 16).
3.4. Image Acquisition
MR imaging was performed using a 1.5 T MRI system (MAGNETOM Vision, Siemens, Avanto, Germany). T1-weighted Turbo-FLASH IR sequence was used for this study. The imaging parameters were TR of 10 ms, TE of 2.06 ms, TI of 830 ms, matrix size of 128 × 128, slice thickness of 10 mm, and number of signal averaging (NSA) of 2. Flip angles were between 10 and 45º (interval of 5º).
3.5. Image Analysis
The image data with DICOM format were transferred from the MR scanner to a personal computer. For data analysis, the image processing software interactive data language (IDL, Research Systems, Inc. http://www.rsinc.com) was used. The programs were written using IDL for the following purposes: 1) Measuring the mean SI of the 9 innermost pixels in the center of each vial for constant and different concentrations; 2) Calculating the correction factors related to coil non-uniformity; 3) Drawing the best-fit of SI versus concentration curves based on equation 3; 4) Finding the maximum linear correlation between the corrected SI and nanoparticles' concentrations at different flip angles with regard to square correlation coefficient (R2) of 0.95 and 0.99.
4. Results
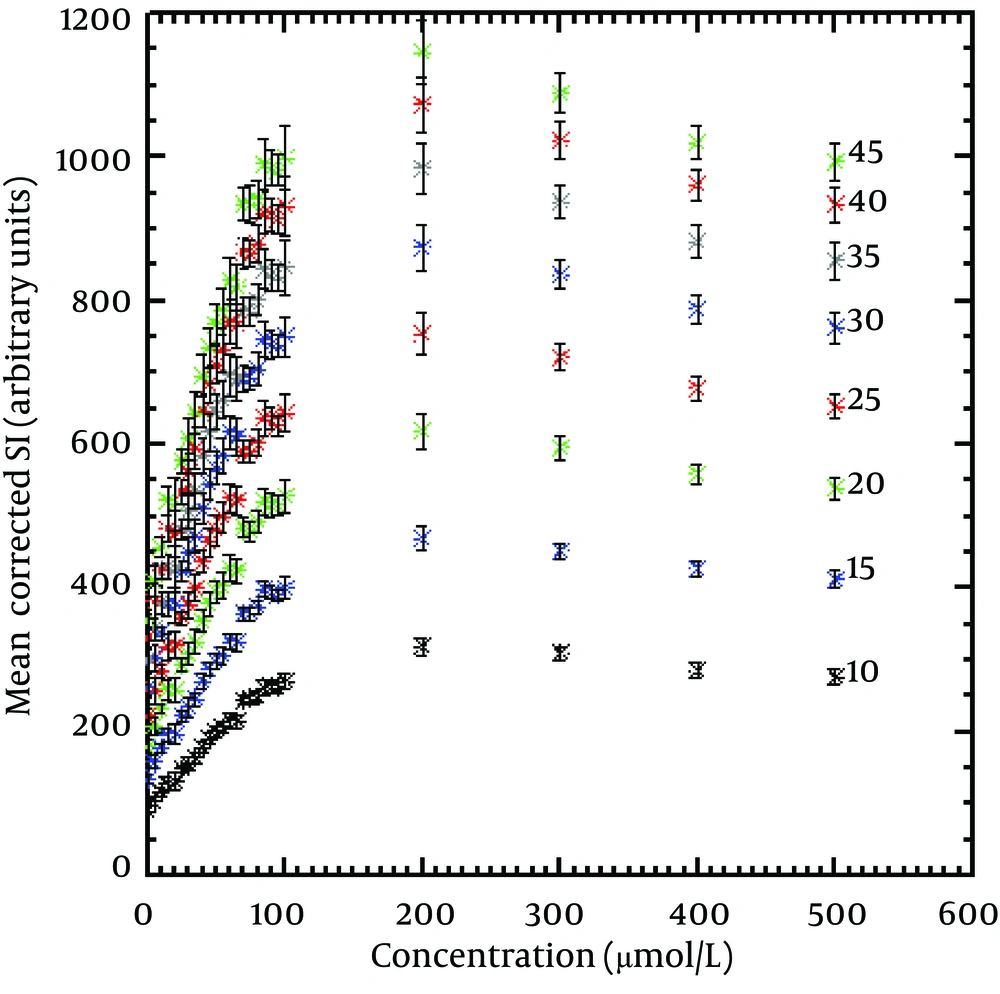
The correction factors for non-uniformity of the coil were calculated for the vial positions with different concentrations. These factors varied from 0.99 to 1.18. The SI of the vials with different concentrations was multiplied by these factors to obtain the corrected SI. Figure 2 shows concentration of the contrast agent versus the mean corrected SI for flip angles of 10-45º. The error bars show the standard deviation of SI from the 9 innermost pixels in each vial. All maximum corrected SIs for different flip angles were seen at a concentration of 200 µmol Fe/L. However, the highest corrected SI was obtained with the highest applied flip angle (45º). After this concentration, the corrected SI was gradually decreased for all flip angles.
The maximum corrected SIs for the applied flip angles are seen in Table 1. The table shows that an increase in the flip angle led to an increase in the maximum corrected SI. Assuming the maximum corrected SI at the highest applied flip angle (45º) is 100%, its value for other flip angles were calculated as Table 1.
| Flip Angle (°) | Maximum Corrected SI | Maximum Corrected SI (%) |
|---|---|---|
| 10 | 326.08 | 28 |
| 15 | 480.50 | 42 |
| 20 | 633.67 | 55 |
| 25 | 772.65 | 67 |
| 30 | 893.48 | 78 |
| 35 | 1006.19 | 88 |
| 40 | 1097.26 | 96 |
| 45 | 1146.59 | 100 |
The maximum linear relationship between corrected SI and the concentrations of the nanoparticles that gives R2 equal to 0.95 and 0.99 for the different flip angles is seen in Table 2. The linear relationship was seen up to 112.21 and 98.83 μmol Fe/L for the short (10°) and the long (45°) flip angles, respectively with R2 of 0.95. These values were reduced up to 48.54 and 42.73 μmol Fe/L for these flip angles with R2 of 0.99. Decreasing the concentration of the nanoparticles that led to the maximum linear corrected SI was seen by increasing the flip angle values.
| Flip Angle (°) | Concentrations of the Nanoparticles that Give R2 = 0.95 (μmol Fe/L) | Concentrations of the Nanoparticles that Give R2 = 0.99 (μmol Fe/L) |
|---|---|---|
| 10 | 112.21 | 48.54 |
| 15 | 109.50 | 47.45 |
| 20 | 108.66 | 46.99 |
| 25 | 106.64 | 46.12 |
| 30 | 105.52 | 45.63 |
| 35 | 103.663 | 44.83 |
| 40 | 101.093 | 43.71 |
| 45 | 98.83 | 42.73 |
5. Discussion
The perfusion parameters such as cerebral blood flow, cerebral blood volume, and mean transit time can be obtained from concentration-time curve after contrast agent administration. MRI measures SI and is unable to measure the concentration of the contrast agent directly. Therefore, it is only possible to achieve SI-time curve with MRI. If the relationship between SI and concentration is to be linear over a range of the concentrations, then relative changes in SI can be used instead of concentration. R2 gives the strength of the linear relationship between SI and concentration. When R2 is 0.99, it indicates that 99% of the variation in SI is explained by the variation of concentration (23).
According to equation 3 and Figure 2, The T1-shortening effect was dominant at low concentrations. Therefore, the relationship between SI and concentration was linear. At high concentrations, both T1 and T2 (with dominance of T2) had effect on the SI response and therefore, it became nonlinear with the concentration. The effect of T1-shortening led to increase in SI, whereas the T2-shortening effect became dominant at high concentrations (more than 200 µmol Fe/L) and led to decrease in SI (Figure 2). The linear parts of all curves were found at the beginning of the curves where the T1-shortening effect was dominant. The linearity of the correlation between mean corrected SI and nanoparticles' concentration decreased as flip angle increased. However, as seen in Table 2, the differences among linearity values for different flip angles were low for both R2 of 0.95 and 0.99. Since R2 indicates the strength of the linear correlation between SI and concentration, the range of the linearity values was lower for R2 of 0.99 than R2 of 0.95.
According to our knowledge, there was no research about evaluating flip angle effect on the linear relationship between SI and different concentrations of iron oxide nanoparticles to compare with the results of this study. As seen in Figure 2Table 1, an increase in flip angle led to an increase in maximum corrected SI based on equation 3. Therefore, among different applied flip angles, the highest corrected SI was seen at flip angle of 45°. At lower flip angles (10-20º), the increase in SI was small due to the increase of the T2-shortening effect. On the other hand, if we assume that the maximum corrected SI resulted by the highest applied flip angle (45º) is 100%, SI for other flip angles will be calculated as shown in Table 1. This finding indicates that the effect of flip angle increase on the maximum corrected SI is relatively higher for low flip angles (14% maximum corrected SI difference between flip angles of 10º and 15º against 4% difference between flip angles of 40º and 45º). This point is important in clinical studies where decreasing of scan time is needed using lower TRs and flip angles. Although the maximum corrected SIs for all applied flip angles were seen at a concentration of 200 µmol Fe/L, there was a considerable difference in the maximum corrected SI when the flip angles varied from 10º to 45º (72 %).
Investigation of the flip angle effect on SI of the blood samples in a T1-weighted gradient-echo sequence before and after administration of USPIO nanoparticles (AMI-227) was carried out by Mayo-Smith et al. (20). They reported that the maximum SI occurred at flip angles of 30º and 60º for before and after administration of AMI-227, respectively. Although the nanoparticle size in the study conducted by Mayo-Smith et al. and our study was approximately similar, the maximum SI in our study was found at flip angle of 45º for both absence of the nanoparticles (concentration of 0) and presence of different concentrations of the particles. The different results can be related to using different imaging parameters and sequences.
Tanimoto et al. (21) evaluated the effect of iron oxide nanoparticles in three-dimensional phase-contrast MR angiography in the rat using flip angles of 20º, 30º, and 40º. They reported the optimal flip angle of 20º for the inferior vena cava before and after USPIO injection. On the other hand, the results of another study (19) showed a little flip angle dependence on the image quality for abdominal MRA using USPIO nanoparticles. Since each material has its own special T1 relaxation time, and the flip angle that gives maximum SI is a function of T1 and TR; therefore, it is not possible to compare other investigators’ results with ours. In addition, our research was an in vitro study and also imaging parameters and sequences in this study were different than those of other investigators. Therefore, our results cannot be compared with their findings.
This study shows that flip angle is one of the important parameters for measuring SI. It is well known that increase of the flip angle leads to increase in maximum SI, but for perfusion measurement it is important to find a flip angle that leads to the maximum linearity at the highest concentration to improve SNR. Since the volume of injected contrast agent in T1-weighted is about 1/10th of the normal suggested dose in T2*-weighted perfusion imaging (23), the use of small volumes of nanoparticles results in reduced SNR. This ratio should be improved using more concentration of contrast agent.
This study also shows that the flip angle has an effect on the maximum linear relationship between SI and concentration. An increase in flip angle leads to a decrease in the maximum linearity (Table 2). Therefore, a flip angle of 10º is the optimum flip angle for perfusion measurement with our imaging parameters and sequence. Since TI = 830 ms in Turbo-FLASH IR sequence is used in clinical studies because of the null point signal from blood, the results of this study may be used for the perfusion measurements (23, 24).
In conclusion, the effect of flip angle on the relationship between SI and different concentrations of iron oxide nanoparticles was evaluated using T1-weighted Turbo-FLASH inversion recovery sequence to find the optimum flip angle that is suitable for perfusion measurement. This study shows that a difference in flip angle has an effect on both maximum SI, and the linear relationship between SI and nanoparticle concentration. The maximum SI will increase at higher flip angles with non-linear relationship between SI and the concentrations of the nanoparticles. The result indicates that an increase in the flip angle leads to a decrease in the range of the linearity. The optimum flip angle that is suitable for perfusion measurement was obtained at 10º for our imaging parameters and sequence. Since this study was performed using a routine 1.5 T MRI system and a TI which suppresses blood signal, the results of this study may be used in in vivo study for perfusion measurements.