1. Background
Colorectal cancer is the second leading cause of cancer-related death in the United States, with approximately 49,700 deaths related to colorectal cancer (1). Rectal cancers account for 30% - 35% of patients with colorectal cancers (2). Various tumor markers and clinical risk factors were reported to be associated with the overall prognosis of rectal cancers, including pre-treatment levels of carcinoembryonic antigen (CEA), pathological T and N stage, tumor differentiation grade, and presence of lymphovascular invasion (3-5).
Recent broad range of treatment options for rectal cancers including increased acceptance of neoadjuvant therapies has enforced the importance of preoperative imaging assessment to determine the best therapeutic option (6). Preoperative imaging modalities for rectal cancer include endorectal ultrasonography (EUS), computed tomography (CT), and magnetic resonance (MR) imaging. In particular, MR imaging is excellent in the diagnosis of tumor infiltration within the bowel wall, lymph node metastases and extramural invasion (7).
Traditional clinical diagnosis for tumor extent and characterization is performed mainly on the basis of conventional MR images acquired using T1-weighted, T2-weighted, and contrast-enhanced T1-weighted sequences. Recently, in order to promote characterization of tumor tissue property, MR imaging protocols have been expanded to incorporate diffusion-weighted MR imaging that assesses the diffusivities of water molecules in tissue. Previous studies reported that apparent diffusion coefficient (ADC) value measured from diffusion-weighted MR images highly correlated with pathological T or N stage, tumor differentiation grade, and extramural depth of tumor in patients with rectal cancer (8-10). However, to our knowledge, no published study evaluated how ADC value is associated with postoperative local recurrence, distant metastasis, and disease-free survival in patients with rectal cancer.
2. Objectives
The purpose of this study was to assess the prognostic ADC value and clinical-pathologic risk factors in patients with rectal cancer.
3. Patients and Methods
3.1. Patients
This retrospective study was approved by our institutional review board and written informed consent was waived. Between January 2010 and December 2013, 94 patients with rectal cancer underwent MR imaging for preoperative tumor staging. Thirty-three of the 94 patients were excluded because of status post neoadjuvant chemotherapy (n = 17), biopsy-only diagnosis with no confirmatory surgical specimens (n = 8), status post radiation therapy (n = 4), preoperative distant metastases (n = 3), and image quality severely degraded by artifacts (n = 1). Thus, the remaining 61 patients (mean age, 64.5 ± 12.1 years; age range, 32 - 86 years), consisting of 41 men (mean age, 65.1 ± 12.5 years; age range, 32 - 86 years) and 20 women (mean age, 63.2 ± 11.5 years; age range, 47 - 83 years) were included in our study cohort.
The interval between the preoperative MR imaging and surgery ranged from 2 to 82 days, with the mean of 19 days. The type of surgery was a low anterior resection in 46 patients, abdominoperineal resection in 13 patients, and local transanal resection in two patients. A total of 62 lesions in 61 patients were histopathologically confirmed as rectal cancer. One patient had synchronous tumors in the rectum. The types of rectal cancer were adenocarcinoma in 60 lesions and squamous cell carcinoma in two.
3.2. MR Imaging Protocol
Pelvic MR imaging was performed using a 3-T MR system (Intera Achieva Quasar Dual; Philips Medical Systems, Netherlands) and a SENSE-Torso coil. The MR protocol included the following sequences: two-dimensional fat-suppressed axial T1-weighted turbo spin-echo imaging (repetition time (TR)/echo time (TE), 759/15 msec; matrix, 464 × 232; field of view, 26 × 26 cm; parallel imaging factor, 1.9; 5-mm section thickness with a 2-mm intersection gap; acquisition time for 20 sections, 2 minute 39 seconds); two-dimensional sagittal T1-weighted turbo spin-echo imaging (TR/ TE, 714/17 msec; matrix, 480 × 240; field of view, 28 × 28 cm; parallel imaging factor, 1.5; 5-mm section thickness with a 2-mm intersection gap; acquisition time for 20 sections, 3 minute 23 seconds); two-dimensional axial T2-weighted turbo spin-echo imaging (TR/TE, 5,894/90 msec; matrix, 512 × 256; field of view, 26 × 26 cm; parallel imaging factor, 1.5; 5-mm section thickness with a 2-mm intersection gap; acquisition time for 20 sections, 3 minute 13 seconds); two-dimensional sagittal T2-weighted turbo spin-echo imaging (TR/TE, 5,625/90 msec; matrix, 512 × 256; field of view, 28 × 28 cm; parallel imaging factor, 1.5; 5-mm section thickness with a 2-mm intersection gap; acquisition time for 20 sections, 3 minute 11 seconds); and two-dimensional axial diffusion-weighted imaging with a single-shot echo-planar sequence (TR/TE, 4,997/60 msec; matrix, 112 × 90; field of view, 28 × 28 cm; parallel imaging factor, 2.3; b factors, 0 and 1,000 sec/mm2; 5-mm section thickness with a 2-mm intersection gap; acquisition time for 20 sections, 1 minute 44 seconds).
3.3. ADC Value Measurement
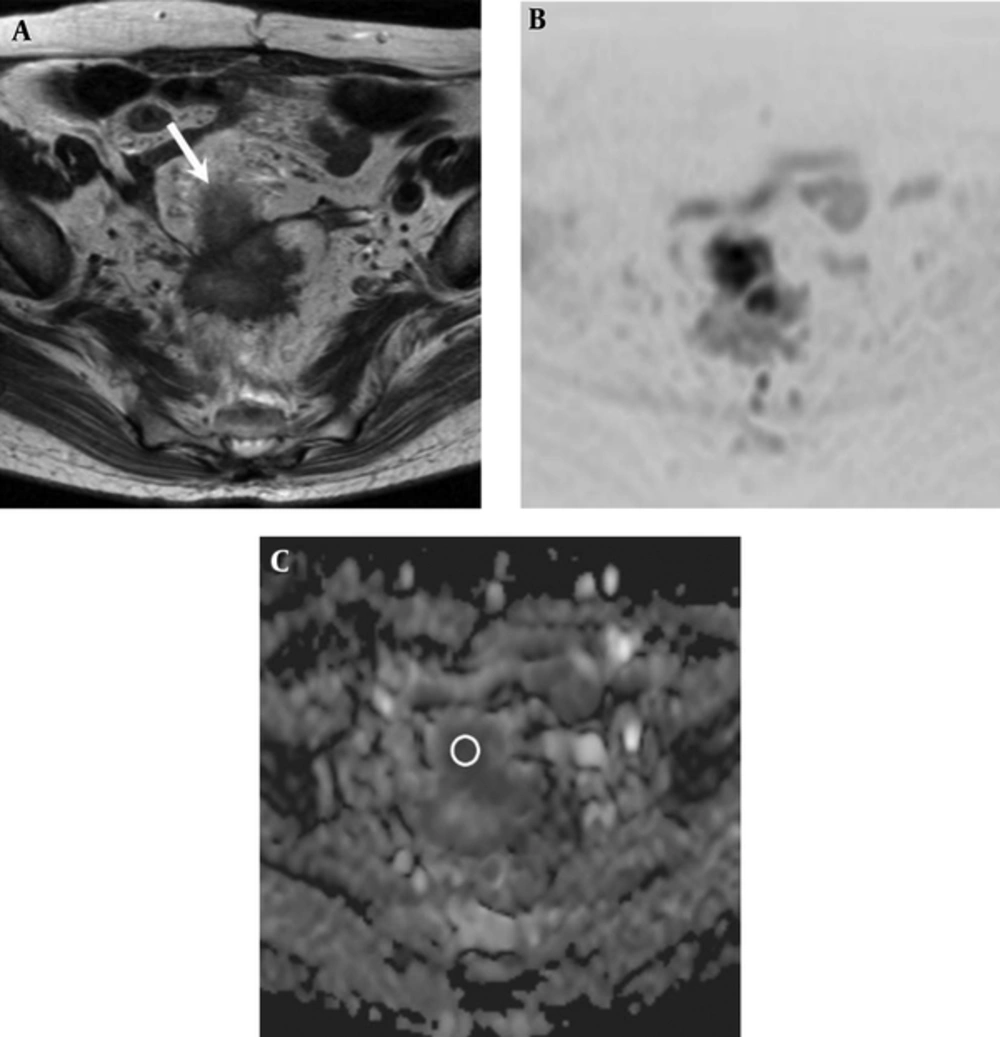
Two experienced radiologists with 6 and 5 years of post-training experience interpreting MR images, who had no knowledge of patient clinical information, measured the ADC value of rectal cancer in consensus. For the ADC value measurement, mean ADC value was obtained by placing a circular region-of-interest (ROI) cursor (37 - 837 mm2). A ROI was drawn to encompass the entire tumor at the image presenting the largest cross-section area of the tumor (Figure 1).
A 74-year-old woman with rectal adenocarcinoma who was diagnosed with lung metastasis 6 months after surgery. A, Axial T2-weighted image shows an ill-defined mass located in the rectosigmoid portion (white arrow). B, Diffusion-weighted image (reversed black-and-white image) and C, Apparent diffusion coefficient (ADC) map show a low ADC value (0.721 × 10-3 mm2/sec) in the mass (circle in part C).
3.4. Prognostic Factors
Various tumor markers and clinical-pathologic risk factors were recorded from the hospital information system at the time of cancer diagnosis. The tumor markers and clinical factors were plasmatic CEA, carbohydrate antigen (CA) 19-9 level, and the presence or absence of postoperative local recurrence or distant metastases. The histological risk factors were pathological T stage (T1, T2, T3, and T4), pathological N stage (N0, N1, and N2), TNM stage (I, II, III, and IV), tumor differentiation grade (1 = well differentiated; 2 = moderately differentiated; and 3 = poorly differentiated), lymphatic (ly0, ly1, ly2, and ly3), and microvascular invasion (v0, v1, v2, and v3). The lymphatic and microvascular invasion were as follow: ly/v0 = no or slight invasion; ly/v1 = mild invasion; ly/v2 = moderate invasion; and ly/v3 = severe invasion.
3.5. Statistical Analysis
Statistical analyses were performed using MedCalc Software for Windows (version 15.8). Fisher’s exact test was conducted to evaluate differences in patient clinical-pathologic risk factors between the patients with and without postoperative local recurrence or distant metastases. An optimal cutoff value that yielded the maximal sensitivity and specificity for the prediction of tumors with postoperative local recurrence or distant metastases was determined using the receiver operating characteristic (ROC) curve. The patients were classified into two groups according to this cutoff value. For each observer measurement, the intraclass correlation coefficient was calculated to evaluate interobserver differences for significance. Other parameters and the cutoff values that we used in the analysis were age (60 years old), plasmatic CEA (5 ng/mL), and CA19-9 (37 U/mL). These plasmatic CEA and CA19-9 cutoff values are based on our institutional standard.
The primary outcome was the disease-free survival, i.e., the length of time from the surgery to the development of postoperative local recurrence or distant metastases. Univariate analysis was performed using the Kaplan-Meier method and log-rank test. Risk factors statistically significant (P < 0.2) from the univariate analysis were reassessed in multivariate analysis that used the Cox proportional hazards regression model. For the subgroup of patients with postoperative local recurrence or distant metastases, disease-free survival was compared between patients below and above the ADC cutoff. A P value less than 0.05 was considered as significant.
4. Results
4.1. Recurrence
Fifty of the 62 lesions (80.6%) presented with no tumor recurrence. The remaining twelve lesions (19.4%) with tumor recurrence were distant metastases in seven lesions, local recurrence in three lesions, and both local recurrence and distant metastases in two lesions. Metastatic sites were lung in three lesions, inguinal lymph node in two, pararectal lymph node in one, liver in one, ovary in one, and peritoneal dissemination in one. The median time to postoperative local recurrence or distant metastases was 11 months (range, 5 - 38 months).
4.2. Patient Background Factors
Table 1 demonstrates the comparison of clinical-pathologic risk factors between the patients with and without postoperative local recurrence or distant metastases. ADC value that yielded the maximal sensitivity and specificity for the differentiation of tumors with and without postoperative local recurrence or distant metastases, was 0.996 × 10-3 mm2/sec. This value was used as the ADC cutoff value. Interobserver reproducibility with the ADC value was substantial agreement (intraclass correlation coefficient: 0.79; 95% confidence interval (CI): 0.65 - 0.87). No significant difference between lesions with and without postoperative local recurrence or distant metastases was observed in terms of patient age (P = 0.50), gender (P = 0.18), plasmatic CEA level (P = 0.32), pathological N stage (P = 0.078), TNM stage (P = 0.36), tumor differentiation grade (P = 0.77), and microvascular invasion (P = 0.11). On the other hand, significant differences were noted in plasmatic CA19-9 level (P = 0.041), pathological T stage (P = 0.031), lymphatic invasion (P < 0.0001), and ADC value (P = 0.0057).
| Variable | With Local Recurrence or Distant Metastasis | Without Local Recurrence or Distant Metastasis | P Value |
|---|---|---|---|
| Age, yb | 0.50 | ||
| < 60 | 5 (41.7) | 15 (30.6) | |
| ≥ 60 | 7 (58.3) | 34 (69.4) | |
| Genderb | 0.18 | ||
| Female | 6 (50) | 14 (28.6) | |
| Male | 6 (50) | 35 (71.4) | |
| CEA, ng/mL | 0.32 | ||
| < 5 | 6 (50) | 34 (68.0) | |
| ≥ 5 | 6 (50) | 16 (32.0) | |
| CA19-9, U/mL | 0.041c | ||
| < 37 | 8 (66.7) | 46 (92.0) | |
| ≥ 37 | 4 (33.3) | 4 (8.0) | |
| Pathological T stage | 0.031c | ||
| 1 | 0 (0) | 6 (12.0) | |
| 2 | 1 (8.3) | 15 (30.0) | |
| 3 | 7 (58.3) | 26 (52.0) | |
| 4 | 4 (33.4) | 3 (6.0) | |
| Pathological N stage | 0.078 | ||
| 0 | 5 (41.7) | 31 (62.0) | |
| 1 | 3 (25.0) | 15 (30.0) | |
| 2 | 3 (25.0) | 3 (6.0) | |
| 3 | 1 (8.3) | 1 (2.0) | |
| TNM stage | 0.36 | ||
| I | 1 (8.3) | 15 (30.0) | |
| II | 4 (33.4) | 15 (30.0) | |
| III | 6 (50.0) | 18 (36.0) | |
| IV | 1 (8.3) | 2 (4.0) | |
| Tumor differentiation grade | 0.77 | ||
| Well | 2 (16.7) | 13 (26.0) | |
| Moderate | 10 (83.3) | 36 (72.0) | |
| Poor | 0 (0) | 1 (2.0) | |
| Lymphatic invasion | < 0.0001c | ||
| 0 | 0 (0) | 1 (2.0) | |
| 1 | 3 (25.0) | 40 (80.0) | |
| 2 | 5 (41.7) | 8 (16.0) | |
| 3 | 4 (33.3) | 1 (2.0) | |
| Microvascular invasion | 0.11 | ||
| 0 | 0 (2) | 7 (14.0) | |
| 1 | 2 (16.7) | 19 (38.0) | |
| 2 | 6 (50) | 18 (36.0) | |
| 3 | 4 (33.3) | 6 (12.0) | |
| ADC value | 0.0057c | ||
| ≥ 0.996 | 3 (25.0) | 36 (72.0) | |
| < 0.996 | 9 (75.0) | 14 (28.0) |
Abbreviations: ADC, Apparent diffusion coefficient; CA, Carbohydrate antigen; CEA, Carcinoembryonic antigen; TNM, Tumor node and metastasis.
aValues are expressed as No (%).
bOverall 61 patients included 12 with local recurrence or distant metastasis and 49 without.
cP < 0.05, significant difference.
4.3. Prognostic Factors
Table 2 demonstrates the results of univariate analysis for the clinical-pathologic risk factors and postoperative local recurrence or distant metastases. Risk factors that were significant in the univariate analysis for the prediction of postoperative local recurrence or distant metastases, included plasmatic cancer antigen (CA) 19-9 level (hazard ratio (HR): 4.15; 95% CI: 0.67 - 25.79; P = 0.010), pathological T stage (HR = 10.16; 95% CI = 1.29 - 80.23; P = 0.027), pathological N stage (HR = 2.67; 95% CI = 0.21 - 33.91; P = 0.0007), lymphatic invasion (HR = 17.95; 95% CI = 1.48 - 218.33; P < 0.0001), and ADC value (HR = 5.75; 95% CI = 1.75 - 18.95; P = 0.0026). Among these factors, plasmatic CA19-9 level (HR = 8.09; 95% CI = 1.99 - 32.87; P = 0.0035), lymphatic invasion (HR = 7.68; 95% CI = 2.04 - 28.95; P = 0.0026), and ADC value (HR = 7.43; 95% CI = 1.78 - 30.98; P = 0.0059) remained statistically significant in the multivariate analysis.
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Age, ya | 0.41 | ||
| < 60 | 1.00 | - | |
| ≥ 60 | 0.62 | 0.18, 2.12 | |
| Gendera | 0.13 | ||
| Female | 1.00 | - | |
| Male | 0.43 | 0.13, 1.48 | |
| CEA, ng/mL | 0.26 | ||
| < 5 | 1.00 | - | |
| ≥ 5 | 1.88 | 0.57, 6.16 | |
| CA19-9, U/mL | 0.010b | ||
| < 37 | 1.00 | - | |
| ≥ 37 | 4.15 | 0.67, 25.8 | |
| Pathological T stage | 0.027b | ||
| 1 | - | - | |
| 2 | 1.00 | - | |
| 3 | 3.58 | 0.93, 13.81 | |
| 4 | 10.16 | 1.29, 80.23 | |
| Pathological N stage | 0.0007b | ||
| 0 | 1.00 | - | |
| 1 | 1.45 | 0.41, 5.21 | |
| 2 | 6.91 | 0.64, 74.47 | |
| 3 | 2.67 | 0.21, 33.91 | |
| TNM stage | 0.47 | ||
| I | 1.00 | - | |
| II | 2.64 | 0.55, 12.61 | |
| III | 4.40 | 0.98, 19.74 | |
| IV | 4.57 | 0.31, 67.17 | |
| Tumor differentiation grade | 0.79 | ||
| Well | 1.00 | - | |
| Moderate | 1.46 | 0.37, 5.85 | |
| Low | - | - | |
| Lymphatic invasion | < 0.0001b | ||
| 0 | - | - | |
| 1 | 1.00 | - | |
| 2 | 6.57 | 1.53, 28.26 | |
| 3 | 17.95 | 1.48, 218.33 | |
| Microvascular invasion | 0.089 | ||
| 0 | - | - | |
| 1 | 1.00 | - | |
| 2 | 3.15 | 0.83, 11.94 | |
| 3 | 4.94 | 0.87, 27.96 | |
| ADC value | 0.0026b | ||
| ≥ 0.996 | 1.00 | - | |
| < 0.996 | 5.75 | 1.75, 18.95 |
Abbreviations: ADC, Apparent diffusion coefficient; CA, Carbohydrate antigen; CEA, Carcinoembryonic antigen; CI, Confidence interval; HR, Hazard ratio; TNM, Tumor node and metastasis.
aOverall 61 patients included 12 with local recurrence or distant metastasis and 49 without.
bP < 0.05, significant difference.
4.4. Disease-Free Survival
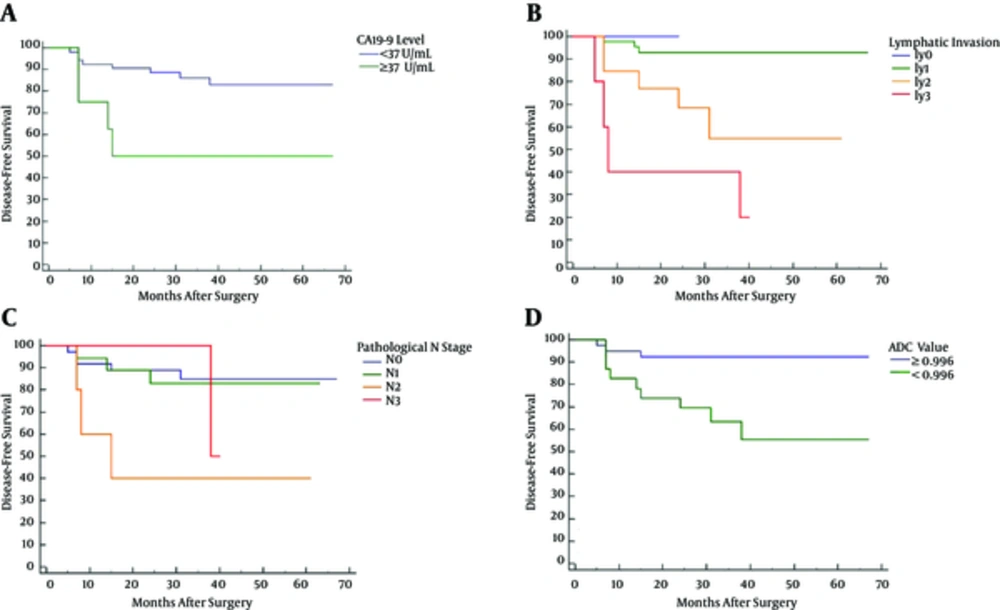
The median follow-up period was 38.5 months (range, 5 - 67 months). Patients with high plasmatic CA19-9 level (≥ 37 U/mL) (P = 0.010), ly2 (P = 0.020) or ly3 (P < 0.0001), pathological N2 (P = 0.006), or low ADC value (< 0.996 × 10-3 mm2/sec) (P = 0.0026) demonstrated lower disease-free survival than those without these markers (Figure 2).
Kaplan-Meier disease-free survival curves regarding plasmatic CA19-9 level (A), lymphatic invasion (B), pathologic N stage (C), and apparent diffusion coefficient (ADC) value (D). High plasmatic CA19-9 level (≥ 37 U/mL) (P = 0.010), ly2 (P = 0.020), ly3 (P < 0.0001), pathological N2 (P = 0.006), and low ADC value (P = 0.0026) were associated with postoperative local recurrence or distant metastases.
4.5. Subgroup Analysis
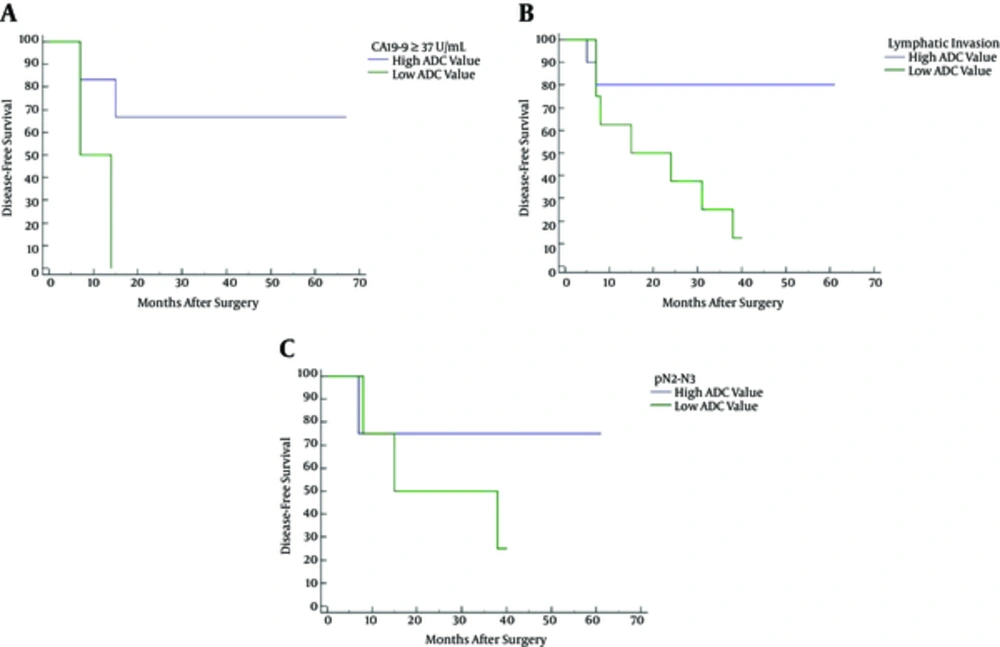
Table 3 summarizes the subgroup analysis results. Two out of eight tumors with high plasmatic CA19-9 level (≥ 37 U/mL) had low ADC (i.e., values below the threshold); whereas, six were above the threshold. Low ADC at the setting of high plasmatic CA19-9 level resulted in reduced disease-free survival (HR: 5.00; 95% CI: 0.36 - 69.34; P = 0.049) (Figure 3A). Eight of 18 tumors diagnosed with lymphatic invasion (ly2 or ly3) showed low ADC values; whereas, 10 were above the threshold. Low ADC at the setting of lymphatic invasion resulted in reduced disease-free survival (HR: 4.66; 95% CI: 1.24 - 17.43; P = 0.030) (Figure 3B). Finally, eight tumors diagnosed pathological N2 or N3 were evenly split, four of them with ADC values below the threshold and four above. Kaplan-Meier disease-free survival analysis showed no statistically significant association (P = 0.60) between high and low ADC (Figure 3C).
| Variable | With Local Recurrence or Distant Metastasis | Without Local Recurrence or Distant Metastasis | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| CA19-9, U/mL | 0.049b | ||||
| ADC ≥ 0.996 | 2 (50.0) | 4 (100.0) | 1.00 | ||
| ADC < 0.996 | 2 (50.0) | 0 (0.0) | 5.00 | 0.36, 69.34 | |
| Lymphatic invasion | 0.030b | ||||
| ADC ≥ 0.996 | 2 (22.2) | 8 (88.9) | 1.00 | ||
| ADC < 0.996 | 7 (77.8) | 1 (11.1) | 4.66 | 1.24, 17.43 | |
| Pathological N stage | 0.60 | ||||
| ADC ≥ 0.996 | 1 (25.0) | 3 (75.0) | 1.00 | ||
| ADC < 0.996 | 3 (75.0) | 1 (25.0) | 2.36 | 0.33, 17.00 |
Abbreviations: ADC, Apparent diffusion coefficient; CA, Carbohydrate antigen; CI, Confidence interval; HR, Hazard ratio.
aValues are expressed as No (%).
bP < 0.05, significant difference.
Kaplan-Meier disease-free survival curves for high versus low apparent diffusion coefficient (ADC) at high plasmatic CA19-9 level (A), lymphatic invasion (ly2 or ly3) (B), and pathologic N stage (pN2 or N3) (C). Low ADC at the setting of high plasmatic CA19-9 level (P = 0.049) or lymphatic invasion (P = 0.030) was associated with decreased disease-free survival.
5. Discussion
In our study, multivariate analysis demonstrated plasmatic CA19-9 level (≥ 37 U/mL), lymphatic invasion (> ly2), and ADC value (< 0.996 × 10-3 mm2/sec) were the significant risk factors for the prediction of postoperative local recurrence or distant metastases in the patients with rectal cancer. Of these four risk factors, plasmatic CA19-9 level and ADC value were the two determined preoperatively. In our study population, pathologically proven TNM stage was not a statistically significant risk factor, which was rather unexpected.
Previous studies reported that pelvic MR imaging was useful for the evaluation of tumor aggressiveness, extramural depth of tumor invasion, and extramural vascular invasion (8-11). ADC value was widely used especially for the evaluation of tumor aggressiveness and correlated with worse prognostic factors, including pathological T stage, plasmatic CA19-9 level, Ki-67 labeling index, and tumor differentiation grade (9, 12). Clinical significance of ADC value is likely associated with the fact that malignant tumors contain a high degree of cellularity and interstitial components such as inflammatory cell infiltration, fibrosis, interstitial edema, tumor necrosis, and mucin. These components hinder free mobility and diffusion of water molecules within tumors, resulting in the decrease of ADC values (13, 14). Tong et al. reported that the ADC value had a significant correlation with extramural depth of tumor invasion in rectal cancer. Authors concluded that the tumor with lower ADC value was associated with more advanced extramural depth of tumor invasion resulting in poorer prognosis (10). We believe that ADC values reflect these tissue components and tumor aggressiveness or disease-free survival.
Our study demonstrated that low ADC values were significant risk factors for postoperative local recurrence or distant metastases in patients with rectal cancer. Although pathological T or N stage and lymphatic invasion are well-known risk factors for postoperative local recurrence or distant metastases, the evaluation of these factors requires a surgical exploration. On the other hand, ADC value can be measured non-invasively prior to surgery.
Traditionally, postoperative chemoradiotherapy (CRT) is recommended for patients with pathological T3 and/or N1-2 tumors (13). Postoperative CRT is also considered for patients with high risk of postoperative local recurrence or distant metastases (involved margins, poorly differentiated grade, and lymphovascular invasion) if preoperative radiotherapy has not been received (14). We postulate that low preoperative ADC value may be an additional evaluating factor for the indication of postoperative CRT, or at least for the recommendation of close follow-up.
Our study had several limitations. First, this study was a retrospective study with a relatively small sample size, which might be subject to a selection bias. Second, ADC values were measured and averaged over an ROI encompassing the entire tumor. Tumor tissue heterogeneity was not considered in the ADC measurements. Third, we did not evaluate the patients who received neoadjuvant chemotherapy or radiotherapy. Finally, the median follow-up period of 38.5 months was relatively short. Further clinical studies with larger sample sizes and longer follow-up periods need to be performed to validate our quantitative data.
In conclusion, tumor ADC values and plasmatic CA19-9 level were the two preoperative biomarkers significantly associated with postoperative local recurrence or distant metastases as well as with disease-free survival in patients with rectal cancer.