1. Background
Breast cancer (BC), the most common malignancy in women (1) and the fifth cause of malignancy death (2), killed 521,000 women, worldwide in 2012 (3). BC outbreaks earlier in some countries compared to the global pattern (4). Life expectancy of BC patients has improved as a result of advanced therapeutic measures. This increased longevity, in turn, has led to observation of more cases who bear post-therapeutic complications (5).
According to BC treatment guidelines, all stage II/III breast cancers are treated by post-operative loco-regional thoracic adjuvant radiation therapy (RT) with or without adjuvant chemotherapy and/or hormonal therapy (6). Side effects of radiation therapy (radiotherapy) depend on the type and volume of the tissue irradiated and the total irradiation dose. Radiotherapy affects different cardiac structures - mainly as fibrosis - and the most common cardiac RT complication is chronic pericarditis (7).
Mortality and morbidity resultant from cardiac disease are increased in patients who undergo RT for BC - particularly, left-sided BC - and coronary artery disease (CAD) is one of the most common causes of non-malignant death in these patients (8-13). A strong correlation between thoracic RT and CAD has been demonstrated (8, 9, 12, 14, 15).
It takes at least 3 years after RT for fibrous plaques to form and about 2 decades for patients to become symptomatic (9, 11, 16, 17). So screening modalities for early atherosclerotic plaque detection would help to improve BC patients’ survival after RT.
Over the past years, coronary CT-angiography (CTA) has developed as an applicable technique in the evaluation of CAD (18, 19). Coronary artery plaque contents are usually calcified and the presence of coronary artery calcification implies CAD. Calcified plaques are detectable on a non-contrasted cardiac CT, usually obtained in conjunction with coronary CTA. The coronary artery calcium score (CACS) is quantitatively measured on non-contrasted cardiac CT images and is a non-invasive screening tool to find CAD in asymptomatic individuals (20).
In the nineties, the new three-dimensional conformal RT (3D-CRT) technique developed to substitute the older conventional two-dimensional RT (2D-RT). In 2D-RT, the X-ray images, tube, and table were used to produce a simulator, the first step of RT planning that simulates the way beams transmit through the patient’s body. By introduction of CT, simulators were designed to visualize the three-dimensional (3D) anatomy of the body. Patients’ 3D images are acquired and in a computer workstation, the whole treatment procedure is virtually planned. Slice-by-slice borders between target volume (to be irradiated), its surrounding organs at risk (for example, heart is radio-sensitive and must be protected in breast radiotherapy), and irradiation planes are defined; so, critical organs receive the least irradiation while neoplastic tissue receives the maximum dose (21). Using 3D-CRT for BC radiotherapy has led to a decrease of irradiation of cardiac tissues (and particularly, the coronary arteries), when compared with 2D-RT (22, 23).
2. Objectives
In this study, we aimed at finding the CACS of patients who underwent loco-regional 3D-CRT as an adjuvant treatment for BC more than three years ago at a referral RT center. Comparing the CACS of the aforementioned patients with non-BC individuals and the patients treated with older RT techniques might determine whether or not the 3D-CRT technique has an effective protective impact on coronary arteries from radiation-induced CAD. The role of CACS as a screening tool in these patients is assessed as well.
3. Patients and Methods
3.1. Study Population
In a prospective research, the study population was selected from women with a history of BC who underwent RT three to nine years ago, referred to a university-affiliated tertiary care center radiation therapy department for follow-up and were evaluated from February 2016 through June 2016. Those who clinically required follow-up thoracic CT scan were enrolled in our study. The exclusion criterion was the presence of proved CAD before the diagnosis of BC. Fifty stage III-A or III-B of BC patients treated by 3D-CRT receiving a total dose of 50 Gy - given in 25 fractions over five weeks - three to nine years ago were enrolled as the case group. All patients underwent modified radical mastectomy followed by chemotherapy. Thirty-two out of fifty patients had received hormonal therapy as well.
Fifty women with no history of BC and/or thoracic RT and no previously proved CAD were enrolled as the control group. They were referred to perform cardiac CTA and CACS, mainly because of atypical chest discomfort.
3.2. Framingham Cardiovascular Risk Score
Systolic and diastolic blood pressures were controlled and the blood sample was obtained for analysis of total cholesterol, high-density lipoprotein (HDL)-cholesterol and fasting blood sugar. Patients were asked to fill out a questionnaire regarding their age, received medications for hypertension, hyperlipidemia, diabetes and presence of a history of cardiovascular disease in their first-degree relatives.
Framingham cardiovascular risk score (24) was calculated for each patient based on gender, age, smoking history, systolic hypertension, hypertension medication, total cholesterol, HDL-cholesterol, and diabetes by using the calculator (25).
3.3. CT Scan and CACS
Non-contrasted thoracic CT scans were obtained using an electrocardiography (ECG)-gating on a 64-slice multislice CT scanner (Brilliance 64; Philips Medical Systems, Cleveland, OH) and reconstructed in 75% of ECG R-R interval of the cardiac cycle to measure CACS. The CACS scan parameters were as follows: patient position: head first; scan length: 135 mm; tube collimation: 40 ×0.625 mm; reconstruction slice thickness: 2.5 mm; rotation time: 400 mSec; field of view: 218 mm; tube potential: 120 kVp; tube current: 196 mA (55 mAs); image matrix: 512 × 512; Ct dose index (CTDIvol): 4 mGy; average dose length product (DLP): 54 mGy × cm and effective radiation dose: 0.96 mSv.
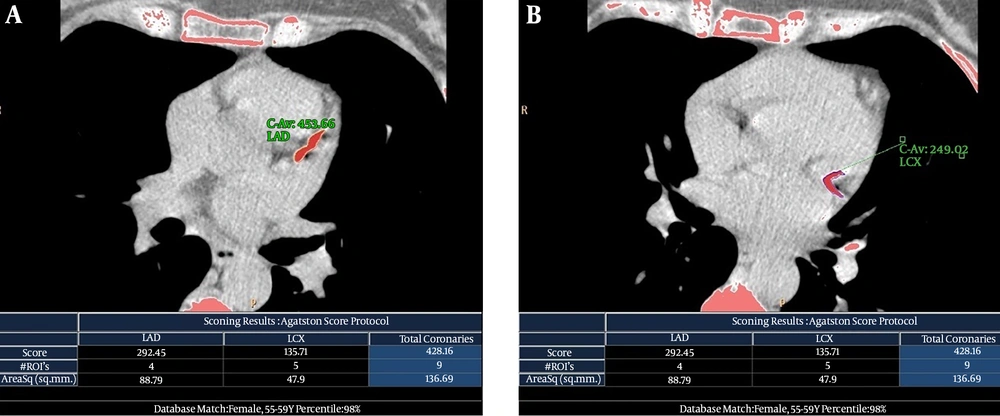
Axial 2.5 mm-thick images from the level of tracheal carina to the level of the diaphragm were reconstructed. The CACS was quantitatively calculated using HeartBeat CS application software available on a diagnostic workstation (Extended Brilliance Workspace; Philips Medical Systems, Best, The Netherlands). The corresponding quantities were semi-automatically calculated as Agatston score (AS) with a density threshold of 130 Hounsfield units for calcified points (26). A 17-segment modified American Heart Association model of coronary artery nomenclature was used to assign major coronary arteries, including the right coronary, left main coronary, left anterior descending and left circumflex arteries (Figure 1).
Non-contrasted electrocardiography (ECG)-gated computed tomography (CT) scans and calculated Agatston score (AS) of a 50-year-old woman (patient number 10 in Table 2) with a history of breast cancer and a 3-year interval between radiation therapy and follow-up study. This patient had a strongly positive family history of coronary artery disease, but no other risk factor was present. A, Long calcified atherosclerotic plaque in the origin of the left anterior descending (LAD) artery with calculated AS of 292.45. B, Long continuous calcified atherosclerotic plaque in the proximal part of the left circumflex (LCX) artery that continues into its first obtuse marginal coronary (OMC) artery with calculated AS of 135.71. The total coronary AS was equal to 428.16.
3.4. Statistics
All analyses were conducted using IBM SPSS Statistics 22 for Windows (IBM Inc., Armonk, NY). The fitness of interval variables to the normal distribution was investigated by one sample Kolmogorov-Smirnov test. Data were described as mean ± SD or median (interquartile range) for interval and count (%) for categorical variables. Comparisons between the case and control groups were made by Student’s t, Mann-Whitney U, Chi-square or Fisher’s exact tests, as needed. Logistic regression models were applied for multivariable analysis. Ρ values of ≤ 0.05 were considered statistically significant.
3.5. Ethical Considerations
All patients had requests to perform CT scans due to clinical reasons. CACS assessment was performed by using minor changes in technical parameters of non-contrasted CT scan and all participants asked to sign a consent. The ethical issues of the study were approved by the Committee for Medical and Research Ethics of Shahid Beheshti University of Medical Sciences.
4. Results
4.1. Patients’ Characteristics
Case group patients were women with the mean age of 50.4 years and median of 51 (ranging 34-70) with a history of BC who underwent mastectomy. The time interval between the radiotherapy and the CACS ranged between 3 years to 9 years at a mean of 5.1 years and a median of 4.5 years post-RT. Twenty-five cases had left-sided BC, while there was right-sided BC in 23 individuals and 2 patients had bilateral BC.
Control group participants were women with a mean age of 50.5 and median of 52.5 (range: 32-74) years without any history of malignancy or radiotherapy.
None of the participants was smoker and there was no clinically-known CAD. One of the patients in case group had a positive family history of CAD in one of her first-degree relatives. None of the women in the control group had such a positive family history of CAD. Other characteristics of case and control groups are summarized in Table 1. Characteristics of BC patients with none-zero CACS are demonstrated in Table 2.
| Case group | Control group | ||||
|---|---|---|---|---|---|
| Rt BC / (interquartile range) | Lt BC / (interquartile range) | Bilat BC / (interquartile range) | Total / (interquartile range) | Total / (interquartile range) | |
| Number of patients | 23 (46) | 25 (50) | 2 (4) | 50 (100) | 50 (100) |
| Positive family history for CAD | 1 (2) | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| HTNb or on medication | 6 (12) | 4 (8) | 0 (0) | 10 (20) | 11 (22) |
| Diabetes mellitus | 1 (2) | 1 (2) | 1 (2) | 3 (6) | 2 (4) |
| Dyslipidemiac or on medication | 12 (24) | 12 (24) | 0 (0) | 22 (44) | 13 (26) |
| Years past after RT | 4 (3-7) | 5 (3 - 6) | 7.5 (6 - 9) | 4.5 (3 - 7) | - |
| AS | 0 (0 - 0.75) | 0 (0 - 3.2) | 0 (0 - 0) | 0 (0 - 1.5) | 0 (0 - 0) |
| Framingham risk score | 4.3 (3 - 7.7) | 5.4 (2.6 - 8.9) | 8.2 (3.1 - 13.3) | 4.9 (2.7 - 8.7) | 6.3 (3.9 - 8.6) |
Abbreviations: AS, agatston score; BC, breast cancer; Bilat, bilateral; CAD, coronary artery disease; HTN, hypertension; Lt, Left; Rt, Right; RT, Radiotherapy
aThe values are presented as No. (%).
bSystolic blood pressure ≥ 140 mmHg and/or Diastolic blood pressure ≥ 90 mmHg.
cTotal cholesterol ≥ 200 mg/dL and/or High-density lipoprotein (HDL)-cholesterol < 50 mg/dL.
| Case | Age | Laterality | Years past RT | HTNa | DM | Dyslipidemiab | Framingham risk for ten years, % | AS | Plaques number | Coronary artery segment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | Lt | 3 | No | No | No | 10 | 5 | 1 | LAD |
| 2 | 58 | Lt | 4 | No | No | No | 7 | 2.62 | 1 | LAD |
| 3 | 50 | Lt | 6 | No | No | Yes | 5.1 | 1.5 | 1 | LAD |
| 4 | 59 | Lt | 9 | No | No | Yes | 7.8 | 63.6 | 3 | LAD:2 LCX:1 |
| 5 | 51 | Rt | 4 | No | No | Yes | 5.4 | 3.2 | 3 | LAD:1 RCA:2 |
| 6 | 70 | Lt | 7 | No | No | Yes | 43 | 6.87 | 3 | LM:1 LAD:2 |
| 7 | 66 | Lt | 3 | No | No | Yes | 8.9 | 12.8 | 1 | LAD |
| 8 | 52 | Lt | 3 | No | No | Yes | 4.7 | 66 | 1 | RCA |
| 9 | 40 | Lt | 4 | No | No | No | 1.59 | 2.3 | 1 | LCX |
| 10c | 56 | Rt | 3 | No | No | No | 6.1 | 428.16 | 2 | LAD:1 LCX:1 |
| 11 | 50 | Rt | 7 | No | No | Yes | 4.1 | 134 | 2 | LCX:2 |
| 12 | 63 | Rt | 3 | Yes | No | No | 8.6 | 15 | 1 | LAD |
| 13 | 55 | Rt | 9 | No | No | No | 3.9 | 193 | 3 | LAD:1 RCA:2 |
Abbreviations: AS, agatston score; CACS, coronary artery calcium score; DM, diabetes mellitus; HTN, hypertension; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main coronary artery; Lt, left; RCA, right coronary artery; RT, radiotherapy; Rt, right.
aSystolic blood pressure ≥ 140 mmHg and/or Diastolic blood pressure ≥ 90 mmHg and/or on hypertension medication.
bTotal cholesterol ≥ 200 mg/dL and/or HDL-cholesterol < 50 mg/dL.
cThe only patient with strong positive family history of coronary artery disease.
4.2. Comparison of Case and Control Groups
Except for dyslipidemia (likely because of the fact that dyslipidemia is commonly found after hormonal therapy), other variables did not show any significant difference between the two groups (Table 3).
| Variable | Case group | Control group | P values |
|---|---|---|---|
| Age | 51 (44 - 56) | 52.50 (41 - 57) | 0.761 |
| DBP | 80 (80 - 90) | 80 (80 - 80) | 0.148 |
| SBP | 120 (120 - 140) | 120 (120 - 130) | 0.072 |
| HTNb | 11 (22) | 10 (20) | 0.806 |
| Dyslipidemiac | 24 (48) | 13 (26) | 0.023 |
| DM | 3 (6) | 2 (4) | 0.646 |
| Positive family history for CAD | 1 (2) | 0 (0) | 0.153 |
| Framingham cardiovascular risk score | 4.9 (2.7 - 8.7) | 6.3 (3.9 - 8.6) | 0.291 |
| AS | 0.0 (0.0 - 1.5) | 0.0 (0.0 - 0.0) | 0.949 |
| Calcified plaque present (AS > 0) | 13 (26) | 12 (24) | 0.817 |
| LM | 1 (2) | 1 (2) | 1.000 |
| LAD | 10 (20) | 11 (22) | 0.806 |
| LCX | 4 (8) | 6 (12) | 0.444 |
| RCA | 3 (6) | 2 (4) | 0.646 |
Abbreviations: AS, agatston score; CAD, coronary artery disease; DM, diabetes mellitus; DBP, diastolic blood pressure; HTN, hypertension; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; SBP, systolic blood pressure; LM, left main coronary artery.
aThe values are presented as No. (%) or Median (interquartile range).
bSystolic blood pressure ≥ 140 mmHg and/or Diastolic blood pressure ≥ 90 mmHg and/or on hypertension medication.
cTotal cholesterol ≥ 200 mg/dL and/or HDL-cholesterol < 50 mg/dL.
4.3. Comparison of Right-sided BC and Left-sided BC
No statistically significant difference was noted (by the exclusion of two patients with bilateral BC) (Table 4).
| Rt BC | Lt BC | P values | |
|---|---|---|---|
| Number of patients | 23 (46) | 25 (50) | |
| Age | 51 (45.5 - 55.5) | 50 (42 - 57) | 0.844 |
| Calcium plaque present | 6 (12) | 7 (14) | 0.686 |
| HTNb or on medication | 6 (12) | 4 (8) | 0.882 |
| Diabetes mellitus | 1 (2) | 1 (2) | 0.952 |
| Dyslipidemiac or on medication | 12 (18) | 12 (26) | 0.773 |
| Years past after RT | 4 (3 - 7) | 5 (3 - 6) | 0.949 |
| AS | 0 (0 - 0.75) | 0 (0 - 3.2) | 0.989 |
| Framingham risk score | 4.3 (3 - 7.7) | 5.4 (2.6 - 8.9) | 0.853 |
Abbreviations: AS, agatston score; BC, breast; Bilat, bilateral cancer; CAD, coronary artery disease; HTN, hypertension; Lt, left; Rt, right; RT, radiotherapy.
aThe values are presented as No. (%) or Median (interquartile range).
bSystolic blood pressure ≥140 mmHg and/or Diastolic blood pressure ≥90 mmHg.
cTotal cholesterol ≥ 200 mg/dL and/or High-density lipoprotein (HDL)-cholesterol < 50 mg/dL.
4.4. Agatston Score
After adjustment for different variables, it was found that only age has a significant correlation with AS (Table 5).
| Coefficient | S.E | P value | Odds ratio | (95% CI) | |
|---|---|---|---|---|---|
| RT history | 0.938 | 1.192 | 0.431 | 2.555 | (0.247 -26.404) |
| Years past after RT | -0.135 | 0.197 | 0.494 | 0.874 | (0.593 -1.286) |
| SBP | 0.010 | 0.020 | 0.607 | 1.010 | (0.972 -1.050) |
| Dyslipidemiaa | 0.743 | 0.614 | 0.226 | 2.102 | (0.631 -7.001) |
| Age | 0.212 | 0.054 | 0.000 | 1.236 | (1.111 -1.376) |
Abbreviations: AS, agatston score; CI, confidence interval; RT, radiotherapy; SBP, systolic blood pressure; S.E, standard error.
aTotal cholesterol ≥ 200 mg/dL and/or High-density lipoprotein (HDL)-cholesterol < 50 mg/dL.
5. Discussion
The cardiovascular disease is the main non-malignant killer of patients after thoracic RT (27). When the irradiated breast is on the left side, the risk of cardiac complications is greater and particularly, the left anterior descending coronary artery receives much more radiation than other coronary arteries (28).
Albeit there is no widely accepted cardiac screening approach for follow-up of post-RT patients, most studies recommend an early cardiac disease evaluation to prevent its progression (9, 11). Most of the previous studies using coronary angiography showed an increased prevalence of stenotic coronary artery plaques in patients who underwent thoracic RT, when compared to the general population, a finding in favor of a causative or aggravating effect of irradiation on atherosclerotic plaque formation (8, 9, 12, 14, 15). Stenotic CAD was reported as a common adverse effect of thoracic irradiation, mostly after conventional 2D-RT (10, 29, 30). CAD starts about three years after RT, but it may take decades to become symptomatic. CACS is one of the recommended non-invasive tests to find coronary artery atherosclerotic plaques, irrespective of risk factors (20, 31). Periodical clinical examinations, laboratory assessments, exercise stress test and imaging screening evaluations (e.g., echocardiography and CACS) are recommended to discover the early findings of cardiac disease in these individuals (32).
Nonetheless, our study of BC patients who underwent 3D-CRT did not demonstrate any higher frequency of calcified coronary plaques in comparison with the concurrently evaluated non-BC women as control group. Our patients had a history of 3D-CRT more than three years ago. Although mean CACS was 5 Agatston units higher in a group of our patients who underwent RT more than five years ago, statistical analysis didn’t show any meaningful difference between them and a group of patients who had a less than five years of post-RT time interval.
To the best of our knowledge, there are two previous studies evaluating CACS burden after thoracic RT for BC patients (33, 34). Chang et al. studied BC patients who were treated using the conventional 2D-RT technique (33, 34). Some of the patients in Tjessem et al. study received radiation by the currently standard 3D-CRT technique; while, their other patients had undergone older 2D-RT (34). All our study post-RT participants underwent 3D-CRT. Similar to our research, none of the two aforementioned studies found a significant correlation between RT and CACS of post-RT patients, when compared to those of general population (35). Moreover, no difference was found between the two RT techniques regarding the severity of resultant coronary artery calcification. Lack of increased CACS in studies conducted by Tjessem et al. and Chang et al. as well as ours is discordant with previous studies that revealed angiographically-detected stenotic plaques in post-RT patients. It is of paramount importance to explain the reasoning behind this discordance.
Some studies proposed that 3D-CRT techniques have shown promise in decreasing the irradiation received by cardiac structures; but, there are limited data on late cardiac events due to the lack of long-term follow-up studies (36, 37). Using older 2D-RT methods, normal tissues are difficult to be appropriately protected due to limited knowledge about the irradiation delivered to each tissue (38).
In contrast to the results found in post-RT BC patients - including ours - two studies on the post-RT Hodgkin’s lymphoma patients showed a strong relationship between RT and CACS (13, 39). Among the studies performed in post-RT (either BC or Hodgkin’s lymphoma) patients, forty-seven Hodgkin’s disease survivors in Andersen et al. study had the longest time interval between RT and follow-up CACS. They found a correlation between the CACS and angiographically-depicted CAD in their patients (39). This may be related to longer time interval between RT and CACS test in their evaluated lymphoma patients and additionally, mediastinal radiation fields in Hodgkin’s disease are larger than those in BC patients. In BC patients just small parts of the anterior cardiac wall and the cardiac apex are exposed to radiation, while in Hodgkin’s lymphoma a larger volume of the heart is irradiated and the coronary artery irradiation is expected to be more extensive. Therefore, the observed difference in CACS of post-RT BC vs Hodgkin’s lymphoma patients may be attributable to different irradiated cardiac tissue volume and/or dissimilar follow-up interval time durations. Engbers et al. reported zero AS in a Hodgkin’s disease patient following RT, while the patient had three-vessel CAD in coronary angiography, implying that atherosclerotic plaques even in post-RT Hodgkin’s lymphoma patients may sometimes be “non-calcified” – similar to BC patients - and thus, not detectable by non-contrasted CACS scans (40).
The major cardiac pathologic processes which occur following RT are inflammation, fibrosis and oxidative stress (32). After RT, the produced free oxygen radicals cause activation of inflammatory cascades which interfere with normal endothelial function. The resultant damage would accelerate atherosclerotic plaque formation (41, 42). When compared to ordinary atherosclerotic plaques, the post-irradiation atherosclerotic plaques contain less lipid, are longer in length and located in ostial part or most proximal segments of the coronary arteries (16). The cause of calcification of some atherosclerotic plaques is not entirely clear. Some genetic predilections were reported to have an impact and likely calcified cholesterol nidus (lipid part of plaques) has a role (43, 44). Also, atherosclerotic plaques after RT contain a lower amount of lipid, implying they are more “sclerotic” than “atheromatous” (27).
It has been shown that the process of calcification of atherosclerotic coronary plaque takes a long time, so that the histological microcalcifications begin to appear when measuring 0.5 to 15 μm, growing to punctate and thereafter, fragmented calcifications, measuring up to 3 mm, in their diameter. The above-mentioned histologically named “punctate” and “fragmented” calcifications are generally called “spotty” calcifications on CT. While growing to a size of 3 mm, they gradually form calcified “sheets” and then “nodular” calcifications, on histological examinations and these two calcified plaque types, measuring larger than 3 mm, are considered as “diffuse” calcifications on CT. Even smaller than 3 mm calcified plaques can be quantified using Agatston score; however, the most reliable measurements are provided when the size of the calcified plaque is 3 mm or more; namely, the diffuse calcification type. In a usual atherosclerotic process, the “punctate” and “fragmented” calcifications are mainly found in middle-age patients, the calcified “sheets” and “nodules” are mostly depicted in the elderly. Therefore, this progression takes many years to be occurred and may strongly influence the results (45, 46).
It has been demonstrated that myocardial perfusion defects found by radionuclide scans in post-RT patients may not follow the coronary arterial territories, a finding that might be attributable to myocardial microvascular disease resultant from irradiation or chemotherapy (47). It seems that high-dose RT leads to atherosclerotic (either calcified or non-calcified) plaque formation in the major coronary arteries; whereas, the lower doses of cardiac irradiation mainly results in microvascular disease (with consequent perfusion abnormalities) and myocardial fibrosis (48).
Framingham’s 10-year risk assessment was performed in our study and no correlation with CACS was found. In Tjessem et al. study, hypertensive patients showed higher CACS, a result not found in our study (43). Our patients, like others, revealed an expected strong increase in CACS with aging, implying an age-related atherosclerotic process in elderly people.
As a conclusion, it seems that in contrast to age-related mostly calcified plaques in atherosclerotic process, the low-fat-content (more fibrous) plaques produced by RT (either 2D-RT or 3D-CRT) in BC patients tend to be less calcified and thus, are less likely to be detectable in CACS scans. Therefore, contrary to the general population, the CACS may be considered as an inappropriate screening test for early CAD detection following RT, since most of the stenotic and clinically-significant plaques would be non-calcified at early post-RT stages. Furthermore, lack of CACS increase in our patients might be related to applied 3D-CRT technique resulting in reduced cardiac irradiation, which may lead to a low rate of aggravated major coronary arterial atherosclerotic - usually bearing calcified plaques - disease.
The most important limitation of our study is its relatively short follow-up period of patients so that some of the plaques might have not enough time to become calcified. As previously discussed, a long time takes for progression from fibroatheromatous plaques to fibrocalcific ones and hence, a relatively short follow-up period of 3 to 9 years may have a remarkable impact on observed results. The other issue of concern is that our study case group does not include all BC patients and was only limited to BC patients who needed thoracic computed tomography for clinical reasons, which may lead to a selection bias. Moreover, as we did not perform coronary CTA in post-RT BC patients, their prevalence of likely non-calcified plaques could not be determined. Furthermore, the assessed control group consisted of patients who were referred for coronary CTA and this, in turn, may result in a selection bias. This was inevitable since symptom-free individuals could not be evaluated by CT (which leads to their unnecessary irradiation) to determine their CACS. Authors recommend further CACS studies on chest CT scan of BC patients just before the start of the RT, which might be performed in their metastasis work-up, to be compared with CACS findings after RT. This may solve the biased results derived by aforementioned selection.