1. Introduction
Solitary fibrous tumor (SFT), an uncommon neoplasm that has been found most commonly in the pleura but may also arise at various extrapleural sites, including the head, neck, lung, abdomen, pelvis, and extremities was first described as a primary spindle-cell tumor of the pleura by Klemperer and Rabin in 1931 (1, 2). SFT in the breast is very rare; only 23 cases have been reported in the medical literature (3-9). Herein, we report a case of breast SFT in a 64-year-old man. We describe the ultrasonographic features of this rare entity in addition to the elastographic findings, which have not been reported in the English literature so far.
2. Case Presentation
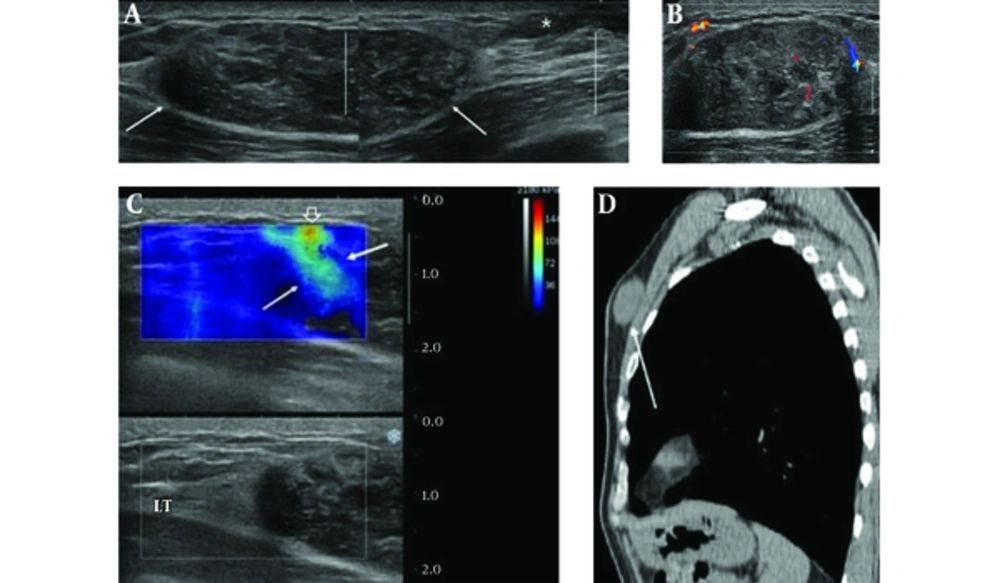
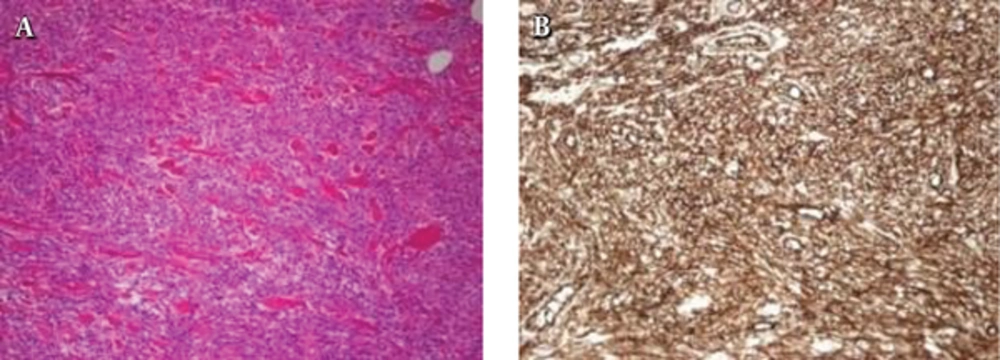
A 64-year-old man presented to our hospital with a painless breast mass. It had been palpable for 10 years, but gradually increased in size over the last two years, causing breast discomfort. He was taking diabetes medication and had no other remarkable medical history or family history of breast cancer. On physical examination, a firm nontender mass measuring about 5.0 × 2.0 cm was noted in the upper inner quadrant of the left breast without overlying skin color change or palpable axillary lymph node. Breast ultrasonography (US) revealed a large solid mass in the upper inner breast 1cm from the nipple. The mass was oval in shape, with circumscribed margins, and had heterogeneous echo pattern with internal vascularity on color Doppler sonography (Figure 1A and B). The mass showed hard elasticity as revealed by shear-wave elastography (SWE) (Figure 1C). Small gynecomastia was observed in the left retroareolar area. The patient had undergone lung cancer screening with low dose chest computed tomography (CT) scan without contrast enhancement a year previously, and the mass had been observed as an isodense solid mass in the left breast (Figure 1D). The long diameter of the mass was slightly increased compared to that observed during the previous screening CT. Considering the elastographic features and size interval change, the lesion was classified as breast imaging and reporting data systems (BI-RADS) category 4. We performed ultrasound-guided core biopsy using a 14-gauge needle. On histological examination, the biopsied tissue revealed fascicles of spindle cells separated by hyalinized collagen bands, indicating the possibility of myofibroblastoma (MFB). The patient underwent complete surgical excision of the mass. On microscopic examination, the resected tumor was composed of bland-looking spindle cells that were arranged haphazardly in the fibrohyaline stroma resembling a keloid (Figure 2A). Immunohistochemical staining showed immunoreactivity for CD34 (Figure 2B), and the cells were negative for S100-protein. On the basis of microscopic findings and immunohistochemical analysis, the final diagnosis was SFT of the breast. The patient had an unremarkable postoperative course.
A 64-year-old man who presented with a painless palpable mass in the left breast. A, Transverse conventional B-mode US image shows a solid mass measuring 4.0 × 1.6 cm with oval shaped, heterogeneously hypoechoic echotexture and well-defined margin (arrows) and small gynecomastia is noted (asterisk). B, The mass has peripheral and intralesional vascularities on color Doppler sonography. C, The mass shows color-coding red in the stiffest part of the mass (open arrow), mixed with light blue (arrows) on shear-wave elastography. D, The sagittal reconstruction of low dose non-enhanced chest CT scan for health checkup that was performed a year ago shows a well-defined oval-shaped solid mass, isodense to the muscle (arrow).
3. Discussion
SFT is a rare mesenchymal neoplasm accounting for < 2% of all soft tissue tumors (3). It most commonly presents during the fourth to seventh decades of life with equal incidence rates by sex (4). It has been known to occur most frequently in the pleural cavity, but it has also been described in other sites - such as the retroperitoneum, abdominal cavity, proximal extremity, and head and neck - which could have occurred owing to the increased recognition of this entity (3). Among the extrapleural sites, SFTs in the breast have only been reported in 23 cases, which is rare (3-9). Moreover, involvement of the male breast is rarer, and only a few cases have been reported (4, 5).
The typical clinical feature of breast SFT is a slowly enlarging nontender mass, and the symptoms differ depending on the location. When the SFT is in the lungs, chest pain and cough may arise. Abdominal pain and distension may be present when the SFT grows in the abdominal cavity. Intracranial SFT can cause headaches, while no other special symptom except a palpable mass is present in a chest wall SFT (10). This was also observed in our case.
The known ultrasonographic features of breast SFT are nonspecific and similar to a benign solid mass, such as a well-circumscribed, oval-shaped, isoechoic, or hypoechoic mass with vascularity (3-8). In our case, the breast SFT was depicted as well-circumscribed, oval-shaped, and heterogeneously hypoechoic mass with peripheral vascularity on color Doppler US.
Elastography, a method for imaging tissue stiffness, is the most noteworthy of the new technologies in recent diagnostic ultrasound systems that improve the specificity for solid masses in the breast. It can help differentiate between benign and malignant masses. According to Barr RG et al., who published the ultrasound elastography guideline, if BI-RADS 3 lesion has characteristics of a malignancy on elastography (strain or shear-wave), such as high stiffness, the lesion should be upgraded and biopsied (11). In our case, the mass showed high shear wave speed color-coding red on the SWE. It was upgraded to BI-RADS 4; therefore, it had to be biopsied. To the best of our knowledge, there have been no articles describing elastographic findings of the breast SFT.
SFT can be hypodense or hyperdense, with respect to muscle depending on the collagen content, with heterogeneous enhancement on CT. On magnetic resonance image (MRI), the SFT is usually isointense on T1-weighted images and variable on T2-weighted images with vigorous enhancement (2). Because of these nonspecific radiologic findings and rarity of the tumor, it is difficult to distinguish SFT from other benign soft tissue tumors. Therefore, the exact diagnosis is usually made after surgical resection and immunohistochemical analysis.
Histologically, SFTs have fibroblast-like spindle-shaped cells and collagen bands. They have high vascularity and a tendency to undergo myxoid degeneration. Differential diagnosis of SFTs includes other bland-looking monomorphic spindle cell lesions in the breast, such as MFB, fibromatosis, nodular fasciitis, hemangiopericytomas, and benign peripheral nerve sheath tumors (8). Among these, MFBs and SFTs share many histological findings, which makes it difficult to differentiate them from each other. However, MFBs have immunoreactivity to muscle antigens, such as a-SMA and desmin, while SFTs have immunoreactivity to CD34 (9). In our case, the pathologic result by core needle biopsy was MFB, but the final diagnosis was SFT based on the detailed immunohistochemical examination. Therefore, small tissue sampling by core biopsy alone may have limited diagnostic value for this rare entity. Recently, nuclear expression of STAT6 (signal transducer and activator of transcription 6, interleukin-4 induced) has been found to a highly sensitive and almost perfectly specific immunohistochemical marker for SFT, and it can be helpful to distinguish this tumor type from histological mimics (12).
Although most SFTs are benign lesions, approximately 10% - 20% of SFTs are malignant, and even benign SFTs have indeterminate malignant potential. Malignant SFTs are correlated with local recurrence and metastasis and the risk of metastasis has been reported to be as high as 25% (3, 4). Histologically malignant lesions are characterized by hypercellularity, with moderate to marked atypia, necrosis, and mitotic activity of more than four mitoses per 10 high-power fields and infiltrative margins (3). Up to now, two malignant breast SFTs have been reported in the literature. These showed clear and focal lobulated boundary on mammography or well-defined margin with pathologic uptake on positron emission tomography with 2-deoxy-2-(fluorine-18) fluoro-D-glucose integrated with computed tomography (7, 13).
In conclusion, we presented a case of breast SFT with a well-defined heterogeneously hypoechoic mass with intralesional blood flows on color Doppler sonography that showed hard elasticity on SWE. Because of the difficulty in its differentiation from other soft tissue tumors due the nonspecific imaging findings and its unpredictable behavior, complete surgical resection with clear margins should be performed, and immunohistochemistry is essential for exact diagnosis.