1. Background
Cerebral venous thrombosis (CVT) is uncommon and accounts for less than 0.5% of all strokes. It occurs from young to old men and women (1, 2). Ferro et al. reported the treatment outcome of CVT patients ranged from complete recovery to permanent neurological deficits (3). Therefore, early diagnosis and treatment are important for the prognosis of the patients. However, CVT is difficult to diagnose because its clinical and imaging findings are diverse and there are many anatomical variations in the cerebral venous system (4). In addition, CVT can progress rapidly and CT attenuation and MR signal intensity change according to the age of the thrombus.
Most of the previous studies have been focused on venous sinus thrombosis (VST). There has been less attention to the isolated or concomitant cortical vein involvement (CVI) of CVT. Because the diameter of the cortical vein (CV) is smaller than that of the venous sinus and there are many anatomic variations in the CVs, CV thrombosis is more difficult to diagnose than VST. Recently, the diagnosis of CV thrombosis has been increasing with the emergence of advanced imaging techniques. According to a recent study of Liang et al. (5), CV thrombosis is common and is associated with severe clinical and radiological manifestations and poor clinical outcomes. However, they have not yet been fully established.
2. Objectives
We aimed to investigate the clinical presentations and radiologic findings of patients with CVT and to evaluate differences in their findings according to the presence or absence of CVI.
3. Patients and Methods
3.1. Patients
Our institutional review board approved this study. Informed consent was waived because of a retrospective study design. From a search of our institutional radiology database between June 2004 and December 2017, forty-nine patients were enrolled in the study. CVT was confirmed in all patients on CT venography (CTV) (n = 40), MR venography (MRV) (n = 15) and/or digital subtraction angiography (DSA) (n = 8) (Table 1).
| Venography | No. (%) |
|---|---|
| CTV only | 29 (59.2) |
| MRV only | 8 (16.3) |
| CTV + DSA | 5 (10.2) |
| CTV + MRV | 4 (8.2) |
| CTV + MRV + DSA | 2 (4.1) |
| MRV + DSA | 1 (2.0) |
| Total | 49 (100) |
Abbreviations: CTV, CT venography; DSA, digital subtraction angiography; MRV, MR venography.
We reviewed patients’ medical records and investigated their clinical symptoms and signs at the initial hospital admission and during the hospital stay. The clinical outcomes of the patients were also assessed using a modified Rankin Scale (mRS) score at discharge.
3.2. CTV Protocol
Bone subtraction CTV examinations were performed on 64-slice CT scanners (Brilliance 64; Philips Healthcare, Best, the Netherlands / LightSpeed VCT; GE Healthcare, Milwaukee, Wisconsin). After the acquisition of nonenhanced CT (NECT) data, contrast-enhanced (CE) CTV was performed. Parameters for the CTV acquisition were as follows: field of view 140 mm, detector collimation 64 × 0.625 mm, table speed 48 mm/rotation, gantry rotation speed 0.75 sec/rotation, kV 120, mA 100 (NECT) and 200 (enhanced CTV), reconstruction section thickness 1 mm, and reconstruction increment 0.5 mm. The scan range included the skull base up to the vertex. A 100 mL bolus of contrast medium (Ultravist 370; Shering, Berlin, Germany) was injected into the antecubital vein at a rate of 4 mL/sec, and the scan delay was determined by using a bolus-tracking technique. A region-of-interest circle was set in the lumen of the aortic arch. When the Hounsfield units in the lumen increased to over 100, the CTV scan was triggered automatically 10 seconds later.
The subtraction process of the NECT and enhanced CTV source data and 3D reconstruction of the CTV images were performed by using commercially available software (Rapidia; Infinitt, Seoul, Korea) (6). The 3D CTV images were reconstructed with maximum intensity projection (MIP). Two series of 36 projected images were generated every 10 degrees around the X- and Z-axes, respectively.
3.3. MR and MR Venography Protocols
MR examinations were performed at 1.5T systems (Magnetom Avanto or Sonata; Siemens Medical Solutions, Erlangen, Germany) or at a 3T scanner (Ingenia; Philips Healthcare, Best, the Netherlands). Imaging sequences included T2-weighted image (T2WI), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and pre- and post-contrast T1-weighted images (T1WIs). Gradient echo T2*-weighted image (n = 8) or susceptibility-weighted image (SWI) (n = 19) was also obtained in 27 patients.
In 15 patients, contrast enhanced three-dimensional MR venography (CE 3D MRV) was also obtained. MRV was performed by using a gradient echo sequence (FLASH 3D, repetition time [TR]/echo time [TE], 3.21/1.23 or 11.0/2.9 ms; slice thickness, 1.4 or 1.0 mm; flip angle, 25 or 30; field of view [FOV], 250 or 220 mm2; matrix, 326 × 384 or 492 × 342; acquisition time, 50 seconds or 1 minute 53 seconds; 1.5T or 3T, respectively). After the acquisition of precontrast scan, CE MRV scan was performed with MR fluoroscopic monitoring (coronal plane at the mid portion of the superior sagittal sinus [SSS]). A bolus of 0.4 or 0.2 mL of gadopentetate dimeglumine or gadobutrol (Magnevist or Gadovist; Schering, Berlin, Germany) per kilogram of body weight was infused intravenously with an injection speed of 3.0 or 1.5 mL/sec. CE MRV scan was started, when the bolus tract of the contrast media arrived in the SSS. After the subtraction process of precontrast and CE MRV source data, 3D MRV images were reconstructed by using a MIP technique. A series of 36 projected images were generated every 10 degrees around the Z-axis.
3.4. Image Analysis
Two radiologists (a neuroradiologist with 25 years’ experience and a 3rd year radiology resident) retrospectively reviewed CT, CTV, MR, MRV and DSA images in a picture archiving and communicating system (PACS). All images were evaluated in consensus without knowledge of the patients’ clinical symptoms and signs.
We evaluated the presence and locations of CVT and accompanying intracranial lesions associated with CVT. The diagnostic criteria of CVT were filling defects in the CV and venous sinus on enhanced CT, CTV, CE T1WI, MRV and/or DSA. In addition, the findings of hyperdensity on NECT, hyperintensity on T1 or T2WI and blooming artifact due to thrombus on SWI in the CV and venous sinus were also considered for the diagnosis of CVT (Figures 1-4) (1, 2, 5, 7). For the evaluation of accompanying intracranial lesions associated with CVT, we assessed the presence and locations of intracranial hemorrhage on CT and/or MR and signal abnormality in the brain parenchyma on MR.
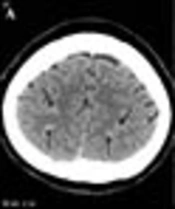
A 53-year-old female with headache and right weakness 3 days before imaging. A, CT scan shows subarachnoid hemorrhage in the cortical sulci of both cerebral convexities (arrows); B, MR venography reveals segmental narrowing in a right cortical vein (arrow); C, On digital subtraction angiography (DSA), there is a filling defect in a right cortical vein (arrow).
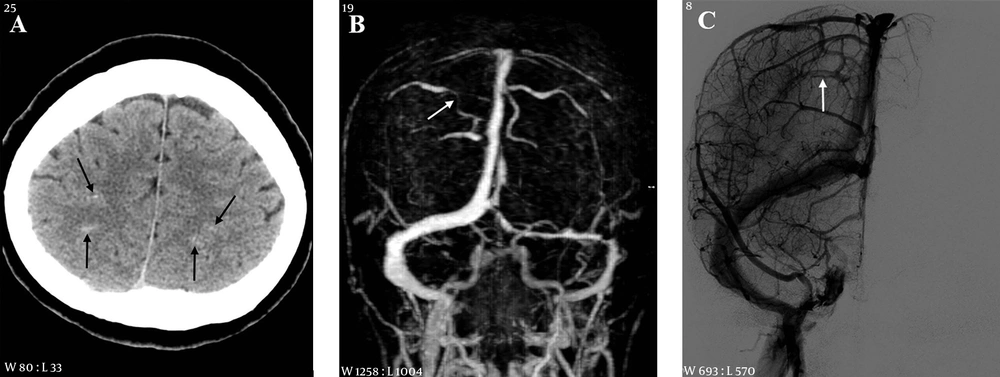
A 38-year-old male with headache, altered mentality and right weakness 2.5 hours before imaging. A, CT scan shows multiple hemorrhages in the left frontal lobe and adjacent cortical sulci (arrow); B, CT scan of the cerebral vertex level reveals hyperdense cortical veins (arrows) at both cerebral convexities; C and D, On CT venography frontal (C) and lateral (D) projection images, the cortical vein (short arrows) and superior sagittal and transverse sinuses (long arrows) are extensively thrombosed.
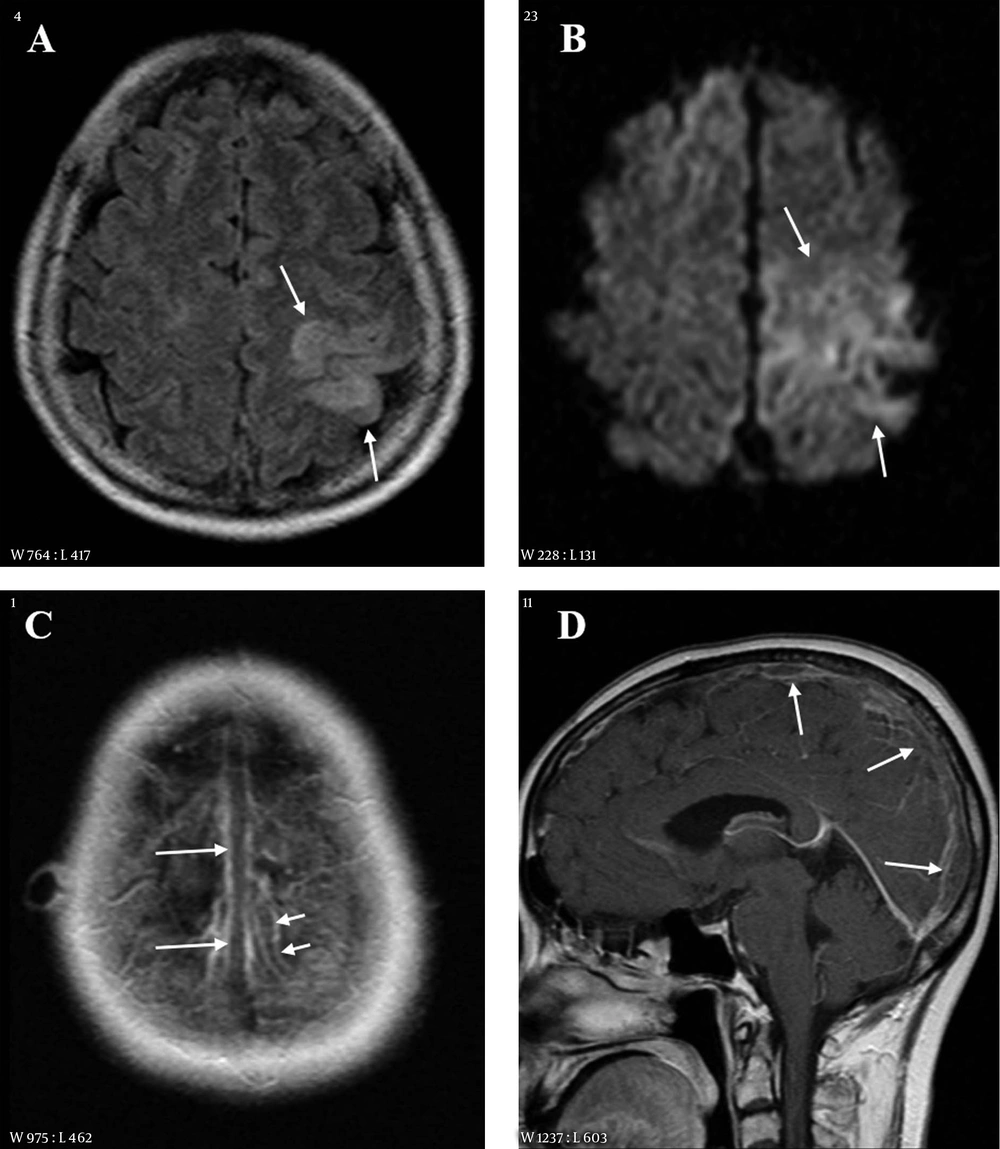
A 55-year-old female with right weakness and seizure 10 hours before imaging. A, Fluid attenuated inversion recovery (FLAIR) image shows a hyperintense lesion in the left parietal lobe; B, Diffusion weighted imaging (DWI) also reveals the lesion as hyperintensity. The extent of lesion is larger than that on FLAIR; C and D, Contrast enhanced axial (C) and sagittal (D) images show filling defects in the superior sagittal sinus (long arrows) and in a cortical vein of the left parietal area (short arrows).
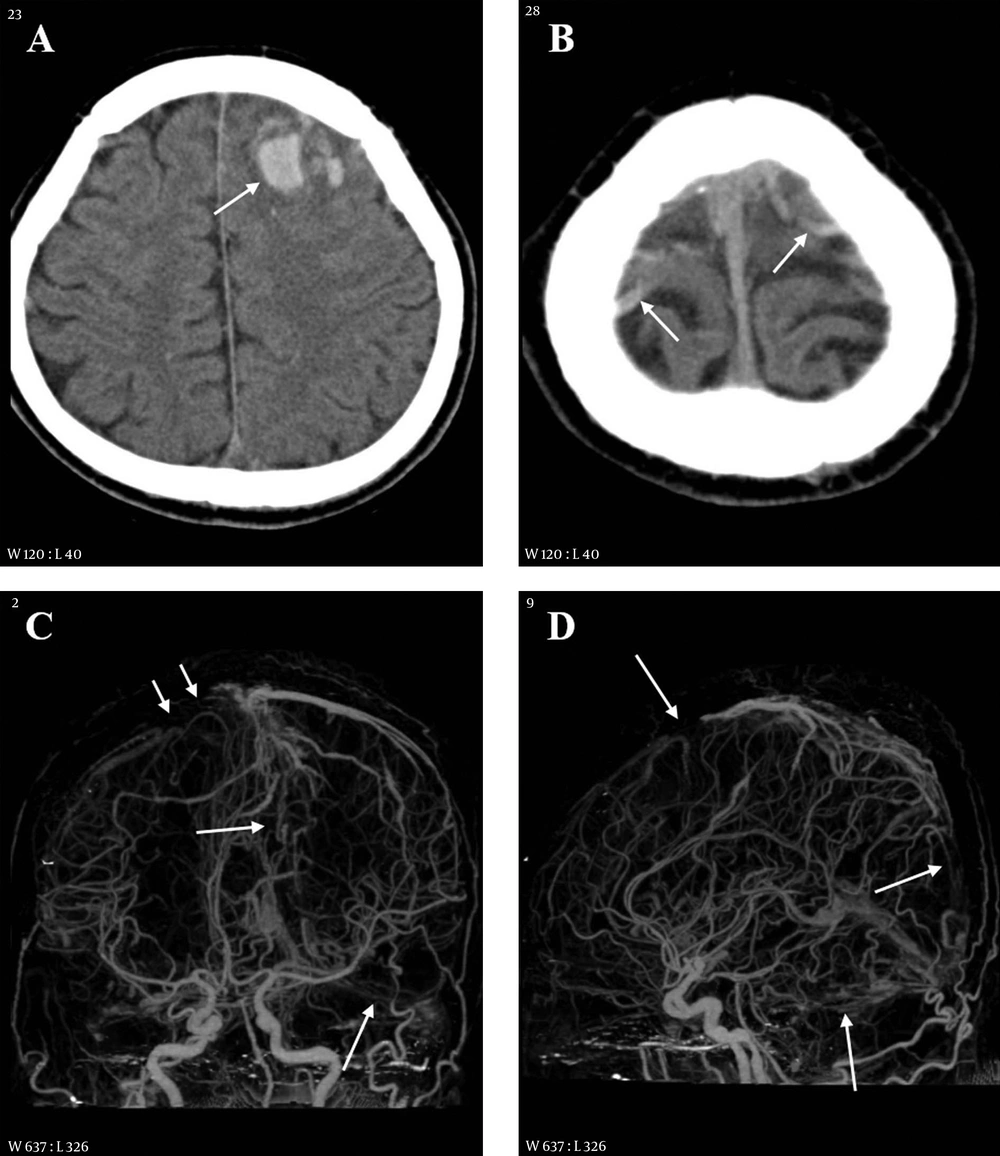
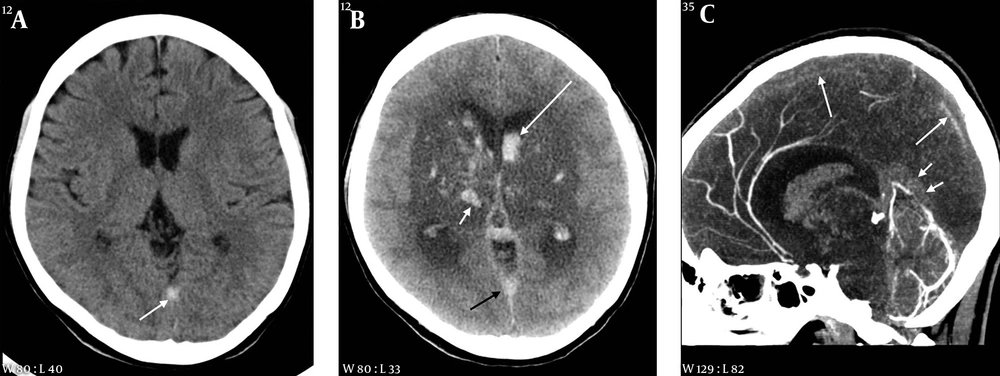
A 35-year-old female with headache and seizure 3 days and 1 hour before imaging, respectively. A, Initial CT scan obtained at an outside hospital shows a hyperdense lesion in the straight sinus area; B, On follow-up CT taken after seizure, there is a large hypodense lesion involving both basal ganglia and thalami. Multiple hemorrhages are associated within the lesion (white short arrow) and in both lateral ventricles (white long arrow). A hyperdense lesion is also seen in the straight sinus area (black arrow); C, Sagittal reformation CT venography image reveals extensive thrombosis in the superior sagittal (long arrows) and straight sinuses (short arrows).
3.5. Statistical Analysis
Regarding the location of the CVT, patients were divided into two groups (CVI positive vs. CVI negative). Statistical analysis was performed to determine the differences in the clinical characteristics and imaging findings between the groups. Statistical analysis was performed for the differences between the groups. The independent samples t test was used for continuous variables, the Mann-Whitney U test for ranked variables, and Fisher’s exact test for categorical variables, respectively. The significance level for the test was P value < 0.05 (2-tailed).
4. Results
The clinical characteristics of the patients are summarized in Table 2. Of the 49 patients with CVT, 22 were male and 27 were female, with an age range from a neonate to 90 years (mean, 48.6 years). Time duration from symptom onset to admission was 30 minutes to 20 days (median, 11.0 hours). Thirty-seven (75.5%) patients visited the hospital within 3 days from the symptom onset, 5 (10.2%) patients between 4 and 7 days, and 7 (14.3%) patients between 8 and 20 days, respectively. The most common symptom or sign was headache (61.2%), followed by focal neurologic deficit (FND) (49.0%), seizure (40.8%), and altered consciousness (18.4%). The involvement sites of CVT are summarized in Table 3. The CV was the most common site (63.3%), followed by SSS (61.2%), transverse sinus (TS) (53.1%), sigmoid sinus (SS) (42.9%), and straight sinus (StS) (20.4%) (Figures 1 - 4). In 35 of the 49 patients (71.4%), the CVT involved two or more sites (Figures 2 - 4).
| Total (n = 49) | CV positive (n = 31) | CV negative (n = 18) | P value | |
|---|---|---|---|---|
| Age, year, mean ± SD | 48.6 ± 20.8 | 48.2 ± 20.5 | 49.4 ± 21.8 | 0.848 |
| Male, No. (%) | 22 (44. 9) | 13 (41.9) | 9 (50.0) | 0.767 |
| Time from symptom onset (hour) median, range | 11.0 (0.5 - 480) | 13.0 (0.5 - 432) | 5.6 (1 - 480) | 0.590 |
| Symptoms and signs | ||||
| Headache | 30 (61.2) | 18 (58.1) | 12 (66.7) | 0.762 |
| Focal neurologic deficit | 24 (49.0) | 21 (67.7) | 3 (16.7) | 0.001 |
| Seizure | 20 (40.8) | 13 (41.9) | 7 (38.9) | 1 |
| Altered consciousness | 9 (18.4) | 4 (12.9) | 5 (27.8) | 0.259 |
| Psychotic symptom | 1 (2.0) | 0 (0) | 1 (5.6) | 0.367 |
| mRS at discharge (median, range) | 0 (0 - 6) | 0 (0 - 6) | 0 (0 - 6) | |
| 0 - 2 | 37 (75.5) | 24 (77.4) | 13 (72.2) | 0.738 |
| 3 - 6 | 12 (24.5) | 7 (22.6) | 5 (27.8) | 0.738 |
Abbreviations: CV, cortical vein; mRS, modified rankin scale; CVT, cerebral venous thrombosis; SD,standard deviation.
| Sites | Total (n = 49) | CV positive (n = 31) | CV negative (n = 18) | P value |
|---|---|---|---|---|
| CV | 31 (63.3) | 31 (100) | 0 (0) | < 0.001 |
| SSS | 30 (61.2) | 23 (74.2) | 7 (38.9) | 0.032 |
| TS | 26 (53.1) | 12 (38.7) | 14 (77.8) | 0.016 |
| SS | 21 (42.9) | 11 (35.5) | 10 (55.6) | 0.234 |
| StS | 10 (20.4) | 3 (9.7) | 7 (38.9) | 0.025 |
Abbreviations: CV, cortical vein; SSS, superior sagittal sinus; TS, transverse sinus; SS, sigmoid sinus; StS, straight sinus, CVT, cerebral venous thrombosis.
aValues are expressed as No.(%).
Among the 49 patients, intracranial hemorrhage was accompanied in 33 (67.3%). The most common location of the hemorrhage was intracerebral (n = 20, 40.8%), followed by subarachnoid (n = 19, 38.8%), subdural (n = 4, 8.2%), and intraventricular (n = 3, 6.1%) (Figures 1, 2, and 4). The subarachnoid hemorrhage (SAH) was located in the cerebral cortical sulci in most cases (Figures 1 and 2). In eight patients, the intracranial hemorrhage was distributed in two or more spaces (Table 4; Figures 2 and 4).
| Findings | Total | CV positive | CV negative | P value |
|---|---|---|---|---|
| Intracranial hemorrhage (n = 49) | 33 (67.3) | 24 (77.4) | 9 (50.0) | 0.063 |
| ICH | 20 (40.8) | 14 (45.2) | 6 (33.3) | 0.550 |
| SAH | 19 (38.8) | 16 (51.6) | 3 (16.7) | 0.018 |
| SDH | 4 (8.2) | 2 (6.5) | 2 (11.1) | 0.618 |
| IVH | 3 (6.1) | 0 (0) | 3 (16.7) | 0.044 |
| Parenchymal hyperintensity (n = 38) | 24 (63.2) | 18 (69.2) | 6 (50.0) | 0.296 |
Abbreviations: CV, cortical vein; CVT, cerebral venous thrombosis; ICH, intracerebral hemorrhage; IVH intraventricular hemorrhage; SAH, subarachnoid hemorrhage; SDH, subdural hemorrhage.
aValues are expressed as No.(%).
On MR, hyperintense lesions in the brain parenchyma, which might be caused by venous congestion or infarction due to CVT, were demonstrated in 24/38 patients (63.2%) on T2/FLAIR or DWI (Table 4 and Figure 3). Of the 24 patients with hyperintense lesions, nineteen (79.2%) showed accompanying intracranial hemorrhage within the lesions and in the cortical sulci adjacent to the lesions [intracerebral hemorrhage (ICH) (n = 9, 37.5%), SAH (n = 7, 29.2%), and ICH + SAH (n = 3, 12.5%)].
Regarding the CVI of 49 patients, the CVI of CVT was demonstrated in 31 (63.3%) patients. Among them, eight patients (25.8%) showed isolated CVI (Figure 1) and twenty-three patients (74.2%) showed concomitant CVI associated VST (Figures 2 and 3). FND was commonly associated in CVI group (67.7 vs. 16.7%, P = 0.001) (Figures 1-3) and SAH was frequent in CVI group (51.6 vs. 16.7%, P = 0.018) (Figures 1 and 2). SSS thrombosis was commonly associated with concomitant CV thrombosis (74.2 vs. 38.9%, P = 0.032) (Figures 2 and 3). However, TS thrombosis showed the opposite result (38.7 vs. 77.8%, P = 0.016). Although overall intracranial hemorrhage (77.4 vs. 50.0%) and MR signal abnormality in the brain parenchyma (69.2 vs. 50.0%) were more common in the CVI group than in the CVI negative group, there were no statistically significant differences. The clinical outcome at discharge was not different between the two groups (Tables 2 - 4).
5. Discussion
In the present study, the most common clinical symptom in our 49 patients with CVT was headache (61.2%), followed by FND (49.0%), seizures (40.8%), and altered consciousness (18.4%), which is consistent with those of previous studies (2, 8-10). The typical pattern of headache was diffuse or progressive pain. CVT may cause the impairment of venous drainage in its tributary, which increases regional cerebral or intracranial pressure and finally induces headache (2, 10). The FND and its severity in our patients varied according to the location, size and extent of accompanying lesions. Although FNDs mostly appear in stroke patients, various diseases including CVT should also be considered for the differential diagnosis in patients with FNDs. The treatment options and prognosis of the patients with CVT are different from those of ischemic or hemorrhagic strokes. Therefore, the differentiation of CVT from strokes is important in clinical practice. Sometimes, it may be difficult to distinguish from acute infarction and diagnosis and treatment can be delayed. Therefore, the application of appropriate imaging techniques and knowledge of their imaging findings are essential for the diagnosis of CVT. The seizure was the third most common symptom and it could be related to the prognosis of patients or not. According to a previous report, seizures in patients with CVT may adversely affect the patients’ prognosis and the mortality increased three times higher than in those without seizures (11). However, other investigators reported a seizure is not a predictor of patients’ prognosis (10, 12). In our patients, we could not find any significant difference in clinical outcomes at discharge between patients with and without seizures.
In our study, cortical SAH and/or ICH were observed in 32/49 patients (65.5%). It was isolated (n = 22, 68.8%) or combined with other space hemorrhage (n = 10, 31.3%). On MR, hyperintense lesions in the brain parenchyma were demonstrated in 24/38 patients (63.2%) on T2/FLAIR or DWI. Among them, ICH was associated within the lesions in 12 patients (50.0%) and SAH was observed in the cortical sulci adjacent to the lesions in 10 patients (41.7%), respectively. The impairment of venous drainage due to CVT increases intravascular pressure in the CVs of its tributary, which induces hemorrhage into the subarachnoid space or brain parenchyma and venous congestion or infarction with/without hemorrhage (2, 10).
Regarding the CVI of CVT in our patients, the CVI was demonstrated in 31 patients (63.3%) and the CV was the most common site of CVT, which is higher than those of previous studies (2, 9, 10, 13). They reported 3.4% - 23% incidence of CVI. However, according to a recent study of Liang et al., CVI was present in 61.4% of their patients (5). The difference in the frequency of CVI in each study might be caused by various factors, which are differences in patient population, imaging techniques, and reader’s experience in imaging interpretation. In our study, the primary venography technique was bone subtraction CTV (n = 40, 82.6%). In 12 patients (24.5%), venography was obtained with two or more imaging modalities. CTV gives a higher spatial resolution than MRV. We also integrated multiple imaging techniques for the detection of CV thrombosis. According to a recent study (14), SWI is useful for the detection of CV thrombosis. In the present study, SWI was obtained in 20 patients. These factors may result the high frequency of CVI in our study.
In the present study, FND and SAH were more common in CVI group than in CVI negative group (67.7 vs. 16.7% and 51.6 vs. 16.7%). SSS thrombosis was frequently associated with concomitant CV thrombosis (74.2 vs. 38.9%). They were statistically significant. Although overall intracranial hemorrhage (77.4 vs. 50.0%) and MR signal abnormality in the brain parenchyma (69.2 vs. 50.0%) were more frequently associated in the CVI group than in the CVI negative group, there were no statistically significant differences. The clinical outcome at discharge was also not different between the two groups. Our findings are partially consistent with those of previous researchers, but there are some discrepancies (5, 13). According to their studies, seizure, FND, altered consciousness, venous infarction, ICH, SAH, and concurrent SSS thrombosis were more frequent in the CVI group than in the CVI negative group (5, 13) and the mRS score at discharge was higher for the CVI group than the CVI-negative group (5). Although we cannot accurately explain why our results differ from those of other researchers, it might be caused by differences in the patient population. Further studies with a large number of patients will be needed.
In conclusion, CVT commonly involved the CV. Although its clinical manifestation was diverse, FND was common in the CVI group. Intracranial hemorrhage and parenchymal hyperintensity were common CT and MR findings and SAH was commonly associated in the CVI group. The knowledge of clinical and radiologic findings and diagnostic suspicion of CVT will be helpful for its early diagnosis and appropriate treatment.