1. Background
Fever and neutropenia are one of the main causes of illness in patients with defective immune systems, especially in people who receive immune suppressive drugs. Fever is defined as a body temperature of more than 38.2°C for more than an hour (1). The lowest normal count for neutrophils in circulation is 1,500 per µL of blood. An increase in the occurrence of infections is seen with decreasing neutrophil counts in the bloodstream, which is intensified when the number of neutrophils reaches less than 500 per µL of blood (2). Neutropenia exists in both acquired and hereditary forms, the former type being common and, most often, a drug-associated phenomenon, especially in association with chemotherapeutics used to treat a variety of cancers and immunological diseases (3). Fungal infections are important causes of death in patients with fever and neutropenia. Common fungal infections include invasive candidiasis and aspergillosis. It is known that immunodeficiency is an important risk factor for fungal infections, and antifungal treatment is a serious affair in controlling the infection. Amphotericin B is the standard treatment for invasive fungal infections, but unfortunately, this drug has side effects such as electrolyte disturbances and nephrotoxicity (4). The emergence of azoles, including micoconazolend, ketoconazole, fluconazole, and itraconazole, opened a new therapeutic strategy to confine aggressive fungal infections (5).
In recent years, new antifungal agents have been developed, including lipid formulations of Amphotericin B (4), azoles (voriconazole and posaconazole), and echinocandin (caspofungin, micafungin, and anidulafungin), as suitable alternatives for treating these infections in patients suffering from neutropenia (6-8). For example, echinocandin is a relatively new class of antifungal drugs inhibiting the synthesis of the fungal cell wall. Caspofungin has activity against candida and aspergillus species and is suitable as the first-line treatment for invasive candida aspergillosis (9). Although the use of antifungal drugs may encounter difficulties and limitations in many cases, it is necessary to use these drugs to improve patients’ clinical conditions and obtain better disease outcomes, especially in patients with invasive fungal infections.
2. Objectives
Numerous studies in developed countries have investigated the effects of experimental antifungal agents in neutropenic patients, but similar studies in Iran are limited. Therefore, the present study aimed to evaluate the experimental results of antifungal treatment in neutropenic patients.
3. Methods
3.1. Ethical Consideration
Before commencing the study, ethical approval was obtained from the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethical code: IR.SBMU.MSP.REC.1398.203).
3.2. Study Population
In this retrospective study, all hospitalized patients in Imam Hussein hospital diagnosed with fever and neutropenia from 2017 to 2018 who received experimental antifungal therapy (including Amphotericin B, Voriconazole, Caspofungin, or fluconazole) were enrolled in the study. The samples were recruited by census and included all patients with neutropenia and fever undergoing experimental treatment with antifungal drugs in Imam Hossein Hospital during 2017 - 2018 (two years).
3.3. Study Procedure
Neutropenic fever was defined as episodes of fever (axillary temperature > 38.2°C or oral temperature > 37.7°C) and neutropenia (neutrophil count < 500 /μL) without the diagnosis of an infection, which was resistant to five days of antibacterial therapy. Demographic information (age, sex), background disease causing neutropenia, laboratory data (mycology testing, biochemical parameters, liver functional tests, and renal tests), antifungal agents administered, side effects, and response to treatment were extracted from patients’ records.
3.4. Statistical Analysis
SPSS version 21 was used for statistical analysis. Data were reported as percentage (%) or mean ± SD. The chi-square test and Fisher’s exact test were used for inferential analysis. It should be noted that a P-value less than 0.05 was considered to identify a statistically significant observation.
4. Results
4.1. Demographic and Clinical Information of Patients
Demographic and clinical information of patients is shown in Table 1. A total of 33 patients were studied. The results showed that leukemia was one of the most common malignancies (AML) in neutropenic patients.
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 17 (52) |
| Female | 16 (48) |
| Total | 33 (100) |
| Underlying disease | |
| CA+ chemotherapy | 6 (18) |
| AML | 15 (45.5) |
| ALL | 3 (9) |
| Lymphoma | 1 (3) |
| CA+ metastasis | 1 (3) |
| DM | 7 (21) |
Demographic and Clinical Information of Patients
4.2. Prevalence of Fungal Infections, Diagnostic Methods, and Drugs Used
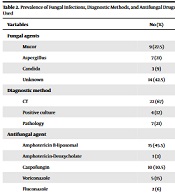
In the present study, fungal infection was confirmed in 19 patients (58%) in the course of treatment. The most common cause of fungal infections was Mucor, followed by aspergillus and candida.
Liposomal Amphotericin B (45.5.9%) and Caspofungin (30%) were the most common active drugs against fungal infections in neutropenic patients (Table 2).
| Variables | No. (%) |
|---|---|
| Fungal agents | |
| Mucor | 9 (27.5) |
| Aspergillus | 7 (21) |
| Candida | 3 (9) |
| Unknown | 14 (42.5) |
| Diagnostic method | |
| CT | 22 (67) |
| Positive culture | 4 (12) |
| Pathology | 7 (21) |
| Antifungal agent | |
| Amphotericin B-liposomal | 15 (45.5) |
| Amphotericin-Deoxycholate | 1 (3) |
| Caspofungin | 10 (30.5) |
| Voriconazole | 5 (15) |
| Fluconazole | 2 (6) |
Prevalence of Fungal Infections, Diagnostic Methods, and Antifungal Drugs Used
4.3. Response to Treatment and Drug Complications
Recovery and mortality after experimental antifungal therapy in patients with neutropenic fever were comparable. In males, the recovery rate was higher than mortality compared to women; but affected women and men showed no significant differences in terms of response to experimental antifungal therapy. In addition, the highest improvement rate (43%) was related to the age group of less than 40 years; however, the difference between various age groups was not statistically significant (P = 0.301) (Table 3).
| Response to Treatment | Total | Recovery | Death | P-Value |
|---|---|---|---|---|
| Total | 33 (100) | 18 (54) | 15 (46) | - |
| Gender | 0.143 | |||
| Male | 17 (52) | 11 (33) | 6 (18) | |
| Female | 16 (48) | 7 (21) | 9 (28) | |
| Age (y) | 0.301 | |||
| < 40 | 14 (43) | 10 (30) | 4(12) | |
| ≥ 60 - 40 | 9 (27) | 4 (12) | 5(15) | |
| ≥ 60 | 10 30 | 4 (12) | 6(18) | |
| Antifungal agent | - | |||
| Amphotericin B-liposomal | 15 (45.5) | 8 (24.5) | 7 (21) | |
| Amphotericin-Deoxycholate | 1 (3) | 1 (3) | 0 (0) | |
| Caspofungin | 10 (30.5) | 4 (12) | 6 (18.5) | |
| Voriconazole | 5 (15) | 4 (12) | 1 (3) | |
| Fluconazole | 2 (6) | 1 (3) | 1 (3) |
Response to Experimental Antifungal Therapy in Patients with Neutropenic Fever a
The rate of response to various antifungals was variable and low in number, not allowing for accurate subgroup comparisons (Table 4).
| Drug Fungus | No. | Amphotericin B-liposomal | Amphotericin Deoxycholate | Caspofungin | Voriconazole | Fluconazole | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rec. | Dec. | Rec. | Dec. | Rec. | Dec. | Rec. | Dec. | Rec. | Dec. | ||
| Mucorales | 9 | 4 | 3 | - | - | 1 | 1 | - | - | - | - |
| Aspergillus | 7 | 1 | 2 | - | - | - | - | 4 | - | - | - |
| Candida | 3 | 1 | - | - | - | 1 | - | - | - | - | 1 |
| Unknown | 14 | 2 | 2 | 1 | - | 2 | 5 | - | 1 | 1 | - |
Response to Treatment Based on Fungi and Antifungals
The frequencies of clinical and paraclinical side effects of experimental antifungal medications are shown in Table 5; however, because of the small and unequal number of the agents, we could not perform subgroup comparisons.
| Drug Side Effect | Amphotericin B-liposomal | Amphotericin-Deoxycholate | Caspofungin | Voriconazole |
|---|---|---|---|---|
| Total | 15 (100) | 1 (100) | 10 (100) | 5 (100) |
| Anorexia | 2 (13.2) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 1 (6.6) | 0 (0) | 2 (10) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 4 (40) | 0 (0) |
| Vertigo | 1 (6.6) | 0 (0) | 0 (0) | 0 (0) |
| Tachypnea | 1 (6.6) | 0 (0) | 0 (0) | 0 (0) |
| Hypoxemia | 4 (26.4) | 0 (0) | 0 (0) | 0 (0) |
| Thrombophlebitis | 0 (0) | 0 (0) | 0 (0) | 1 (20) |
| BUN↑, Cr↑ | 10 (66.6) | 0 (0) | 4 (40) | 2 (40) |
| K↓ | 12 (80) | 1 (100) | 2 (20) | 0 (0) |
| Bicarbonate↓, Mg↓ | 7 (46.6) | 0 (0) | 0 (0) | 0 (0) |
| ALT↑, AST↑ | 7 (46.6) | 0 (0) | 0 (0) | 0 (0) |
| Thrombocytopenia | 7 (46.6) | 1 (100) | 0 (0) | 0 (0) |
| Leukopenia | 6 (40) | 1 (100) | 0 (0) | 0 (0) |
| Anemia | 15 (100) | 1 (100) | 10 (100) | 5 (100) |
| Hemolysis | 1 (6.6) | 0 (0) | 0 (0) | 0 (0) |
| Coagulopathy | 1 (6.6) | 0 (0) | 0 (0) | 0 (0) |
Frequency of Clinical and Paraclinical Side Effects of Antifungal Agents a
Reduction of creatinine clearance (42%) and hypokalemia (39.4%) were the most common complications, respectively (Table 5). The most common clinical and laboratory complications were related to liposomal Amphotericin B and Caspofungin, respectively. It should be noted that these two drugs were used more frequently than others.
There was no significant difference in the side effects of experimental antifungal therapy in patients with neutropenic fever based on age groups and gender (P > 0.05).
There were two heart failures, one in a deceased 75-year-old man with EF = 20% treated with Caspofungin. The other case occurred in a recovered 63-year-old woman with EF = 40% on Voriconazole. There were no clinical or paraclinical side effects in the only patient treated with Fluconazole.
5. Discussion
Fever and neutropenia are among medical emergencies and the causes of hospitalization in patients with immunodeficiencies, especially in people receiving immunosuppressive drugs. Neutropenia is the most important primary risk factor for infections. In neutropenic patients, due to decreased immune responses, fever may be the first and sometimes the only sign of infection. Various studies have reported the highest prevalence of neutropenic fever in patients with different types of malignancies (10, 11). In the study of Aguilar-Guisado, AML (44%) and lymphoma (27%) were the most common underlying diseases in neutropenic patients (11). In line, our results also showed that AML was the most common underlying disease in patients with neutropenic fever.
Infections are among the major causes of death in neutropenic patients. Neutropenia and impaired phagocytic defense predispose to bacterial and fungal infections, which are major causes of fever in neutropenic patients (12, 13). Death due to fungal infections in neutropenic individuals often occurs in about 80% of cases, and more than 90% of fungal infections are caused by Candida and Aspergillus species (14); however, the rate of infections caused by rare species, such as Trichosporon, Pseudellescheria, Fusarium, and Scedosporium, is increasing in a worrying manner (15-17). In our study, fungal infection was confirmed in 19 patients (57.5%). Mucors species were the most common causes of fungal infections (24.5%), followed by Aspergillus (21%) and Candida (12%). In 14 (43.5%) patients who remained, no fungus was found. In Barreto et al.’s study, invasive fungal infections in neutropenic patients with AML and myelodysplastic syndromes were confirmed to be caused by Mucor and aspergillus species, each in 2 (50%) cases (18). In another study, Candida and Aspergillus were reported as the most common fungi causing infections (19). In a study by Aguilar-Guisado et al., Aspergillus fumigatus and Sedosporium were identified as definitive infectious agents in patients with neutropenic fever (11). Differences between studies can be a result of variable patient and hospital conditions, as well as the diagnostic methods used to identify fungal agents. However, in most studies, candidiasis, aspergillosis, and mucormycosis have been reported as the most common fungal infections in patients with neutropenia, which should be considered when choosing experimental antifungal medications for these patients.
Early detection of the cause of infection in neutropenic patients and administering appropriate treatment play a key role in reducing mortality and cutting financial costs for the individual and society. Since it is not possible to detect the source of the infection and the organism responsible for the infection in most cases, experimental treatment regimens have been used for these patients for many years (20, 21). In the present study, Liposomal Amphotericin B (45.5%) and Caspofungin (30.5%) were the most commonly used antifungal agents during the experimental treatment of neutropenic patients, similar to many other studies (10, 11, 19, 22). In this study, experimental antifungal therapy in patients with neutropenic fever led to a 47% recovery rate and 53% mortality rate, showing no significant difference in total and according to gender and age groups. The results of most studies are similar to that of our study (10, 11, 19, 23). The experimental treatment, which is decided based on clinical criteria, risk factors, and diagnostic approaches, can be used as an effective approach and an alternative to general experimental treatment for managing patients with persistent fever and neutropenia. In addition, in the present study, the highest response rates to antifungal therapy in neutropenic patients were related to Liposomal Amphotericin B and Caspofungin, respectively. Studies have reported different results in terms of response to treatment in neutropenic patients (6, 24-27). Responses to experimental antifungal therapy in neutropenic patients may rely on their clinical conditions and the type of and resistance to fungal agents.
In the present study, the most common side effects of experimental antifungal therapy in neutropenic patients included a decrease in creatinine clearance (42.4%) and hypokalemia (39.4%), with Liposomal Amphotericin B and Caspofungin showing the most common side effects. In a study by Cordonnier et al., the results showed that creatinine clearance was reduced during experimental antifungal treatment (19). Different side effects have been reported in patients receiving antifungal agents (6, 19). Therefore, in experimental antifungal therapy in patients with neutropenic fever, the use of effective antifungal agents with fewer side effects should be considered.
5.1. Conclusions
In the present study, Mucor, Aspergillus, and Candida species were the most common causes of fungal infections in patients with neutropenic fever. Experimental antifungal therapy in neutropenic patients led to equal rates of recovery and mortality. Therefore, our study emphasizes that experimental treatment in neutropenic patients should be selected based on clinical criteria and risk factors and with a diagnostic approach to replace general experimental treatments.