1. Introduction
There are approximately 2% of people with Meckel’s diverticulum, the most common congenital anomaly of the gastrointestinal tract (1). There is no doubt that Meckel’s diverticula are true diverticula since they contain all the layers in the small intestines (1). It is typical to find them within 100 cm of Ileocecal valve (1). According to one large study, the distance from diverticum to the Ileocecal valve, in children under two years of age was 34 cm, 46 cm for three to 21 years old, and 67 cm for adults over 25 years of age (2). In Meckel’s diverticula, 60% of mucosa is heterotopic, of which 60% is gastric mucosa (1). Other common tissues include Brunner’s gland, pancreatic islets, colonic mucosa, endometriosis and hepatobiliary tissue (1). In most cases, Meckel’s diverticulum is asymptomatic unless there are complications associated with them (1). The lifetime complication rate for patients with Meckel’s diverticulum is estimated to be approximately 4 - 6% (3). The most common symptoms of Meckel’s diverticulum are bleeding, intestinal obstruction, and diverticulitis (1). In adults with Meckel’s diverticula, intestinal obstruction is the most frequent symptom (1). In Meckel’s diverticulum, adhesions or intussusception (4), volvulus, mesodiverticular band entrapment, and strictures caused by chronic diverticulitis are common obstructers (1). It is extremely rare for Meckel’s diverticulum, Meckel’s primary enterolith, and mesodiverticular band to cause acute obstruction and perforation of small intestines. Delay in diagnosis and treatment may severely affect the outcome. Here, we report such a rare case of small bowel obstruction as well as the literature review.
2. Case Presentation
A 65 years old male with no known co-morbidities reported a history of 16 days of severe abdominal pain associated with abdominal distention, bilious vomiting, and obstipation. Initial non-operative management at a private hospital was unsuccessful. He denied a history of abdominal tuberculosis or any major illnesses in the past. He also denied any past history of abdominal surgery.
On physical examination, the patient is thinly built with body mass index (BMI)-19.6 kg/m2. His abdomen was distended, and he had rebound tenderness in his right lower quadrant. Bowel sounds on auscultation were sluggish. External hernial orifices were normal, and digital rectal examination findings were unremarkable. His pulse rate and blood pressure were 94/54 mmHg and 130/min, respectively, according to his vital signs. He was afebrile but dehydrated. Pallor was present. He had no Icterus. Nasogastric aspirate was feculent.
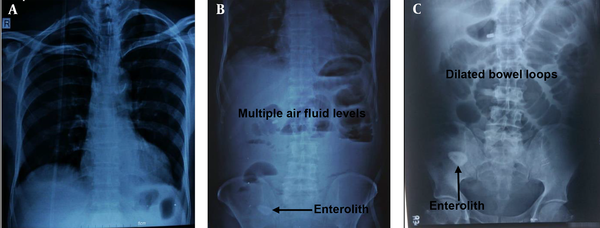
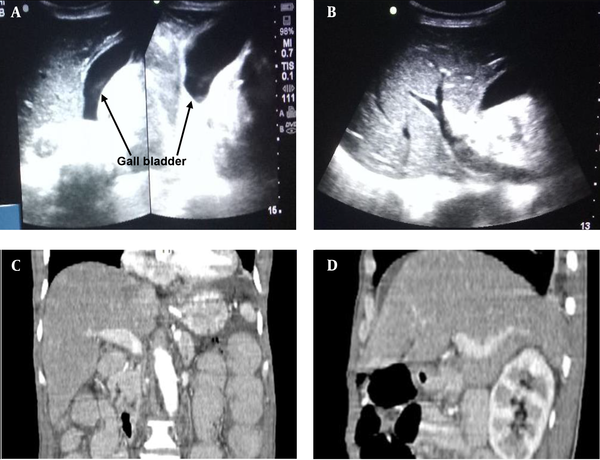
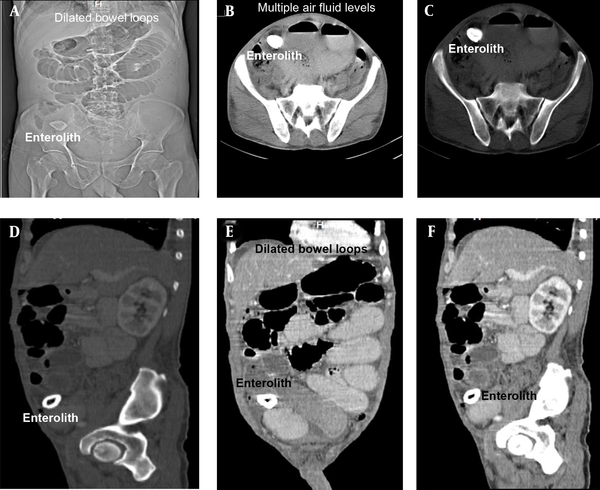
The laboratory investigation findings revealed neutrophilia (total leukocyte count of 21,100/mm3 with neutrophils of 94%). The patient had normal renal and liver function results except for slightly raised serum amylase and lipase. His hemoglobin value was 9.8 gm/dL. Moreover, X-ray abdomen and chest revealed dilated small bowel loops and three cm oval to round shadow at right iliac fossa (Figure 1). Ultrasonography (USG) abdomen (Figure 2 A - B) and contrast-enhanced computed tomography (CECT) abdomen (Figure 2 C - D) suggested small bowel obstruction with a normal hepatobiliary system. CECT abdomen with intravenous and oral contrast revealed features suggesting proximal small bowel obstruction and enterolith (Figure 3 D - F).
X-ray chest with dome of diaphragm 1A, showed no-gas-under-diaphragm, X-ray abdomen (erect and supine); 1B-C, showed dilated jejunum, proximal and mid ileum and multiple air-fluid levels. A radio-opaque-shadow (stony density) was seen in right iliac fossa (RIF). A, X-ray chest PA view; B, X-ray abdomen (erect); C, X-ray abdomen (supine).
USG and CECT abdomen show normal hepatobiliary and portal anatomy without any evidence of obstruction, wall erosion or destruction, focal lesion or any bilioenteric fistula. These proved that the described stone in our case report was a primary enterolith and it did not originate from gall bladder; A, transabdominal USG showed empty GB lumen, normal CBD, normal portal vein and biliary radicals, no evidence of aerobilia or obstruction; B, transabdominal USG showed no direct or indirect evidence of bilio-enteric fistula (aerobilia), no evidence of erosion or destructions of wall; C, CECT abdomen (coronal plane) showed enlarged liver (17.5 cm). No evidence of any focal lesion was seen. GB distended. No intra luminal calcific density seen; D, CECT abdomen (sagittal plane) showed normal portal radicals and hepatic veins. Biliary radicals and CBD are not dilated. No aerobilia was observed.
Images of CECT abdomen, showing small bowel obstruction with intra-luminal calculus in distal ileal loop right iliac fossa region in the zone of transition; A, scout film with stone in right-iliac-fossa; B, axial post-contrast CT: Intra-luminal stone with multiple air-fluid levels; C, axial post-contrast CT: Intra-luminal stone with multiple air-fluid levels; D, CECT abdomen (sagittal), showing enterolith with peripheral hyperdense calcification with hypodensity center; E, CECT abdomen (sagittal), showing dilated Jejunal, proximal and midileal loops (maximum caliber 4.5 cm); F, CECT abdomen (sagittal), showing collapsed distal ileal loops.
2.1. Management
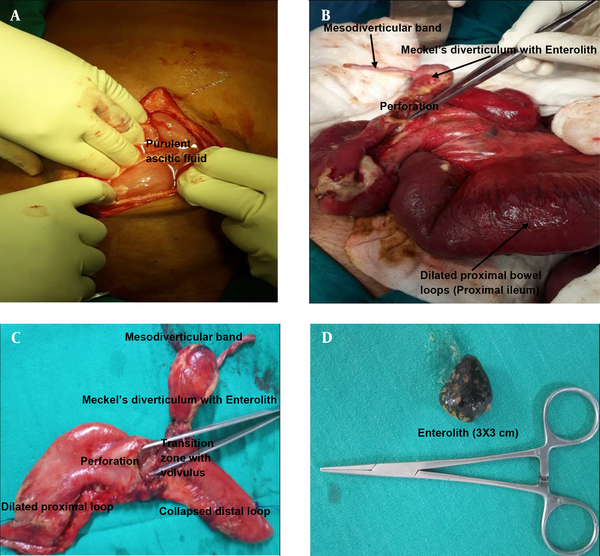
Following resuscitation, the patient was taken up for emergency laparotomy. Intra-operative findings (Figure 4) revealed purulent ascetic fluid, dilated small bowel loops, and collapsed terminal ileum with transition zone in between. Meckel's diverticulum was present at the transition zone and located 26 cm from Ileocolic junction. Meckel’s diverticulum had a 3 × 3 cm calculus and a fibrous mesodiverticular band from the tip to the adjacent mesentery, causing localized entrapment of small bowel loops. As a result of Meckel’s diverticular band and primary enterolith, the ileum was twisted along the long axis of the band and diverticulum. The transition zone was formed by the twisted base of Meckel’s diverticulum and adjacent ileum. Also, 1.5 × 1.5 cm localized perforation was seen at or just proximal to transition zone. Gall bladder, liver, and the rest of viscera were normal.
Intra-operative Images and specimen images showing enterolith in perforated Meckel’s diverticulum with mesodiverticular band. Meckel’s-diverticulum was found at 26 cm from ileocolic junction (unusual location). No bilio-enteric fistula was observed intra-operatively; A, purulent ascitic fluid on opening the peritoneum. The peritoneum was thickened; B, dilated proximal loops with perforation at the base of Meckel’s diverticulum with mesodiverticular band. Picture was taken after adhesiolysis and correction of volvulus; C, Meckel’s diverticulum with mesodiverticular band with perforation at its base; D, enterolith from Meckel’s diverticulum.
Adhesiolysis was carried out and untwisted the ileal volvulus. Resection of 10 cm segment of ileum along with Meckel's diverticulum was done, and primary anastomosis was carried out (side to side, four layers, hand-sewn). Drain was placed, and abdomen closed. Parenteral nutritional was provided in immediate postoperative period. Early oral feeding commenced as soon as the patient passed flatus as per enhanced recovery after surgery (ERAS) protocols. Patient was discharged to home on postoperative day-10. HPE report confirmed Meckel’s diverticulum with perforation. No heterotrophic mucosa was seen (Figure 5).
Postoperative images of the patient and wound. Postoperative period was uneventful; A, post-operation day 01. Patient started ambulation. Chest physiotherapy and incentive spirometer started; B, patient and wound on post-operation day 08. Wound was healthy and healing. Staples were in place.
3. Discussion
The most common congenital anomaly of the gastrointestinal tract is the Meckel’s diverticulum which is a true diverticulum, involving the whole walls of the small intestine. John Friedrich Meckel described the embryological basis for congenital diverticulum of the midgut in 1809 (5). Gurvits and Lan reported that 3 - 10% of cases with Meckel’s diverticulum are complicated by enterolithiasis (6). It has been reported that approximately 50 cases with Meckel’s diverticula have enterolith (7). According to a study on 1,476 patients with Meckel’s diverticulum, enterolithiasis was observed using laparotomy in 0.7% of asymptomatic patients and 6% of symptomatic patients (8). Differential diagnosis for enterolithiasis includes calcified abscess, possibly caused by Crohn’s disease, an ingested foreign body, phytobezoar, trichobezoar, lactobezoar or pharmacobezoar, calcified neoplasm, undescended testicle and teratoma (6).
Various complications can result from an enterolith, such as obstruction (6), diverticulitis (6), injury to the bowel mucosa (6), perforation (6), afferent loop syndrome (9), intussusception (10), gangrene (6), hemorrhage (6), and iron deficiency anemia. An enterolith-related intestinal obstruction due to Meckel’s diverticulum has rarely been reported (11). A diverse range of obstruction can exist besides enterolith, including the trapping of a bowel loop by a mesodiverticular band, the volvulus of diverticulum around the band, and intussusception (1, 12). As the yolk sac atrophies, one of the vitelline arteries degenerates, while the other develops into the superior mesenteric artery (13). A peritoneal-covered fibrous band forms when one of the vitelline arteries fails to degenerate, and this peritoneum-covered fibrous band is known as mesodiverticular band (13).
Kuru et al. (14) also reported a small bowel obstruction that was caused by mesodiverticular band. However, acute small bowel obstruction due to both mesodiverticular band and primary enterolith in Meckel’s diverticulum is rarely seen. In our case during pre-operative evaluations, the patient was anemic, but he denied any history suggesting lower gastrointestinal bleeding. Rectal examination findings were unremarkable. USG abdomen and CECT abdomen showed distended GB without any stones inside the lumen (Figure 2). There was no direct or indirect radiological evidence of bilioenteric-fistula (Figures 2 and 4). These proved that the described stone in our case report was a primary enterolith, and it did not originate from gall bladder.
Enterolith in our case report showed peripheral calcifications and radiolucent center on X-ray and CT (Figures 1 and 3), which again proved that stone was primary enterolith. Similar findings of peripheral calcifications and radiolucent center were also shown in another study by Higginson and Hall (15). Thus, both primary enterolith in Meckel’s diverticulum and a mesodiverticular band were the causes of acute small bowel obstruction and perforation in our case. The prolonged stasis might be the cause of enterolith formation in Meckel’s diverticulum. To the best of our knowledge, our case is the first case of its type, involving both Meckel’s primary enterolith and mesodiverticular band in causing acute small bowel obstruction and perforation. In such a scenario, an exploratory laparotomy was a life-saving measure. Although the rest of the bowel was healthy, resection and anastomosis were carried out. We followed early oral feeding for the patient as per ERAS protocols during post-operation.
3.1. Conclusions
It is extremely rare for Meckel’s diverticulum, Meckel’s primary enterolith, and mesodiverticular band to cause acute obstruction and perforation of small intestines. It requires prompt evaluation and exploratory laparotomy. Prompt evaluation and treatment may significantly affect morbidity and mortality. Hence, our case report underlines that combined involvement of Meckel’s primary enterolith and diverticular band in causing acute bowel obstruction is extremely rare, but these can be one of differential diagnosis for small bowel obstruction.