1. Background
Healthcare has grown into one of the largest industries worldwide, and the Emergency Department (ED) stands out as a crucial department within these services that exhibits significant demands (1). The ED is fully prepared and equipped to deliver comprehensive emergency care to the community during emergencies and non-emergencies. Operating round the clock, 365 days a year, this department operates uniquely, involving multiple interactions and requiring intensive decision-making. These factors can result in interruptions and disruptions within this section (2).
Over the past few decades, overcrowding in hospital ED has become a widespread issue across the globe. The rise in patient numbers and the influx of patients requiring admission have exacerbated this problem. Substantial evidence suggests that overcrowding has detrimental consequences, such as prolonged wait times for critically ill individuals, reduced patient satisfaction, heightened mortality rates, and increased medical errors (3).
Efficiently distributing resources in the healthcare industry and enhancing societal health quality is paramount. However, given the constraints of limited resources, the high expenses associated with healthcare, and the sensitive and complex nature of the field, it has consistently remained contentious (4). Hence, to effectively allocate resources to patients, it is necessary first to diagnose the deterioration of their condition. Conversely, early identification and prevention of untimely deaths using extensive clinical data present a significant challenge for emergency physicians, requiring substantial expertise and precise intuition (5).
Artificial intelligence (AI) refers to the capacity of computer programs to perform tasks or reasoning processes typically associated with human intelligence. Its main focus is on making accurate decisions despite ambiguity, uncertainty, or the presence of large data sets. In the healthcare domain, where extensive amounts of data exist, machine learning (ML) algorithms are utilized for classification purposes ranging from clinical symptoms to imaging features. Machine learning is a methodology that leverages pattern recognition techniques. Within the clinical field, AI has found applications in diagnostics, therapeutics, and population health management. Notably, AI has significantly impacted areas such as cell immunotherapy, cell biology, biomarker discovery, regenerative medicine, tissue engineering, and radiology. The application of ML in healthcare encompasses drug detection and analysis, disease diagnosis, smart health records, remote health monitoring, assistive technologies, medical imaging diagnosis, crowdsourced data collection, and outbreak prediction, as well as clinical trials and research (6).
Several conventional methods are used in clinical settings to assess the condition and predict the mortality risk of intensive care patients. These methods include the Simplified Acute Physiology Score (SAPS II), Sequential Organ Failure Assessment (SOFA), and Acute Physiological Score (APS). They incorporate factors such as age, medical history, vital signs, and laboratory test results. These scoring systems help healthcare professionals determine the severity of a patient's illness and predict life-threatening events like sepsis, cardiac arrest, or respiratory arrest (7). Barboi et al. (8) indicated that ML models exhibit higher accuracy than traditional scoring models. Therefore, clinicians are encouraged to prioritize the selection of models that have undergone more rigorous validation.
Li et al. (5) demonstrated that ensemble models, specifically bagging and boosting, exhibit superior performance compared to single classifiers. By analyzing demographic and laboratory data from 1,114 ED patients, the researchers found that the gradient boosting machine (GBM) model stood out with an impressive accuracy rate of 93.6% in predicting patient mortality.
In a retrospective cohort study conducted by van Doorn et al. (9), the accuracy of predicting patient outcomes in the ED differs when utilizing only laboratory information compared to a combination of laboratory and clinical data. Specifically, the study employed the Extreme Gradient Boosting (XG Boost) model and found that when using solely laboratory information, the accuracy was 82%. However, by integrating both clinical and laboratory data, the accuracy increased to 84%. The study involved 1,344 ED patients.
Klug et al. (10) used variables including age, admission mode, chief complaint, five primary vital signs, and emergency severity index (ESI) to analyze ED patients. By implementing the XG Boost model, the study achieved an impressive accuracy rate of 92%. The ESI is a tool used in ED to assess the severity of a patient's condition and prioritize care accordingly (11).
2. Objectives
This study aimed to accurately predict patients' mortality within the ED while also conducting a comparative evaluation of different models. By achieving high forecasting accuracy, this study aimed to provide doctors and ED specialists with valuable insights to prioritize patients effectively regarding resource allocation.
3. Methods
The Cross Industry Standard Process for Data Mining (CRISP-DM) is a process model designed for data mining that can be applied across various industries. This model encompasses six sequential phases, executed iteratively from understanding the business requirements to the final deployment and implementation of the data mining solution (12).
To conduct our study, we gathered the electronic health records, medical data, and demographic information of 1,000 patients who were admitted to the ED of a hospital in Tehran. The data were retrospectively collected using the Hospital Information System unit during a one-month timeframe.
We initially removed patients with missing data from the study during the data preparation phase. Additionally, we employed the Interquartile Ranges (IQRs) to detect and eliminate outliers. As a result, 200 patients were excluded from the complete dataset. The IQR is a measure of statistical dispersion that quantifies the spread of a data set. It is defined as the difference between the third quartile (Q3) and the first quartile (Q1) in a data set (13). We employed a label encoder for the target column to represent binary categories, where class 0 signifies discharged, and class 1 signifies expired. After considering the research by Newaz et al. (14), which explored the model's accuracy with over-sampling and under-sampling, we concluded that over-sampling would be the most suitable approach for balancing the classes in the target column.
Feature selection is a crucial step in analyzing data as it involves selecting a concise group of pertinent features. The RF classifier serves as a critical foundation for wrapper algorithms, effectively addressing all significant issues by offering a measure of variable importance (15). To prevent overfitting, RF feature selection was employed. Expert judgment was utilized to eliminate features with an importance score below 0.0095. Subsequently, the models were created using the remaining features. In the modeling phase, the decision was made to use ensemble models due to their relatively good accuracy.
Ensemble models combine multiple models that work together to make predictions. These models can be of the same type or different types, and by leveraging the strengths of each individual model, ensemble models can often outperform any single model. Ensemble models have become popular in various domains, including machine learning and data science because they can improve the overall performance and robustness of a prediction system. They reduce bias and variance, increase model generalization, and mitigate the risk of overfitting. By aggregating the predictions from multiple base models, ensemble models can capture a wider range of patterns and improve the accuracy of predictions (16). The commonly used ensemble techniques are bagging, boosting, and stacking (17):
- Bagging involves training multiple decision trees on various subsets of the same dataset and then averaging their predictions.
- Boosting, on the other hand, works by sequentially adding ensemble members that improve upon the predictions of prior models, ultimately resulting in a weighted average of all predictions.
- Stacking involves training multiple models of different types on the same data and utilizing another model to learn the most effective way to combine these predictions.
The RF algorithm is a widely known supervised ML technique used in both classification and regression problems. This algorithm leverages a collection of decision trees, each trained on different subsets of the dataset, and combines their predictions through averaging to enhance the overall predictive accuracy. This approach, known as bagging, has contributed to the algorithm's popularity. Notably, empirical studies have shown that the Random Forest (RF) classifier outperforms individual classifiers regarding classification rates. Furthermore, it demonstrates shorter training time than Decision Tree and SVM algorithms (18).
Cat Boost (CB) is a GB framework developed by Yandex, a Russian search engine company. It is specifically designed to work with categorical features in the dataset and provides superior performance compared to other traditional gradient-boosting models. Cat Boost can automatically handle categorical features without requiring explicit feature engineering or encoding, making it a convenient choice for working with datasets containing categorical variables. It uses a novel algorithm called "Ordered Boosting" that reduces the impact of the order of categorical features on model performance (19).
Some key features of CB include (20):
- Handling of categorical features
- Improved accuracy
- Fast training time
- Robustness to outliers
Hence, we employed RF and CB models in this study to predict mortality and assess their relative efficacy.
In the evaluation phase, accuracy, precision, recall, and the F1-score are essential criteria for evaluating classification problems. These metrics are calculated as follows (21):
A true positive
K-fold cross-validation is a popular technique used in ML to evaluate the performance of a model on a limited dataset. It helps estimate how well the trained model performs on unseen data. In K-fold cross-validation, the dataset is divided into k equal-sized subsets or folds. The model is then trained on k-1 folds and tested on the remaining fold. This process is repeated k times, each time using a different fold as the test set and the remaining folds as the training set. The model's performance is averaged over all k iterations to obtain a more reliable estimate (22). To ensure a more precise assessment, we employed 5-fold cross-validation.
The receiver operating characteristic (ROC) curve visually depicts how well a binary classifier system performs as its threshold for decision-making is adjusted. It is commonly used in data mining and ML to assess the classifier's performance. The area beneath this curve serves as a measure to evaluate the classifier, and a higher area indicates a better-performing model (23).
4. Results
After completing the data preparation phase, 800 patient records were ready for review and model building. The research findings revealed that 63.88% of these patients were men. Additionally, 18.36% of the overall data was observed to reflect cases of patient mortality.
To ensure a more thorough analysis, we decided to separate the numerical features from the binary features. We then analyzed their statistical characteristics separately for two specific scenarios - discharges and deaths. Employing a confidence level of 95%, we conducted t and chi-square tests. This led us to compile Table 1, which contains statistical information related to the numerical features, and Table 2, which shows statistical information related to the binary features.
| Features | Description | Outcome | P-Value (0.05) | t-Test | |
|---|---|---|---|---|---|
| Discharged (Class 0) | Expired (Class 1) | ||||
| Age, y | Patient's age | 64.56 ± 12.3 | 67.28 ± 13.1 | 0.017 a | -2.381 |
| HB | Hemoglobin | 11.91 ± 2.2 | 11.59 ± 2.2 | 0.127 | 1.526 |
| TLC | Total leukocytes count | 12.27 ± 5.8 | 17.42 ± 13.6 | <0.001 a | -4.481 |
| Platelets | Thrombocytes | 242.43 ± 102.6 | 208.12 ± 120.05 | 0.002 a | 3.210 |
| Glucose | Carbohydrate | 179.75 ± 88.1 | 194.20 ± 108.6 | 0.087 | -1.715 |
| Urea | Body waste | 57.03 ± 39.7 | 86.18 ± 53.5 | <0.001 a | -6.227 |
| Creatinine | Creatinine | 1.56 ± 1.3 | 1.89 ± 1.05 | 0.004 a | -2.859 |
| BNP | B-type natriuretic peptide | 888.32 ± 921.6 | 1370.18 ± 1203.6 | <0.001 a | -4.562 |
| EF | Ejection fraction | 37.89 ± 12.6 | 30.40 ± 8.3 | <0.001 a | 8.799 |
a Statistical significance at a P-value of 0.05.
| Features and Description | Outcome | P-Value (0.05) | Chi-square Test | |
|---|---|---|---|---|
| Discharged (Class 0) | Expired (Class 1) | |||
| Gender | 0.332 | 0.941 | ||
| Female | 241 | 48 | ||
| Male | 412 | 99 | ||
| Smoking | 0.003 a | 8.798 | ||
| No | 599 | 145 | ||
| Yes | 54 | 2 | ||
| Alcohol | 0.001 a | 10.284 | ||
| No | 593 | 145 | ||
| Yes | 60 | 2 | ||
| Diabetes (DM) | <0.001 a | 26.713 | ||
| No | 308 | 104 | ||
| Yes | 345 | 43 | ||
| Hypertension (HTN) | 0.001 a | 11.970 | ||
| No | 310 | 93 | ||
| Yes | 343 | 54 | ||
| Coronary artery disease (CAD) | 0.003 a | 8.798 | ||
| No | 228 | 74 | ||
| Yes | 425 | 73 | ||
| Cardiomyopathy (PRIOR CMP) | <0.001 a | 24.491 | ||
| No | 463 | 73 | ||
| Yes | 190 | 74 | ||
| Chronic kidney disease (CKD) | 0.006 a | 7.675 | ||
| No | 569 | 115 | ||
| Yes | 84 | 32 | ||
| Raised cardiac enzymes | 0.009 a | 6.810 | ||
| No | 471 | 90 | ||
| Yes | 182 | 57 | ||
| Severe anemia | 0.365 | 0.819 | ||
| No | 642 | 146 | ||
| Yes | 182 | 1 | ||
| Anemia | 0.091 | 2.858 | ||
| No | 509 | 105 | ||
| Yes | 144 | 42 | ||
| Stable angina | 0.243 | 1.361 | ||
| No | 647 | 147 | ||
| Yes | 6 | 0 | ||
| Acute coronary syndrome (ACS) | 0.004 a | 8.798 | ||
| No | 375 | 65 | ||
| Yes | 278 | 82 | ||
| St elevation myocardial infarction (STEMI) | 0.938 | 0.006 | ||
| No | 526 | 118 | ||
| Yes | 127 | 29 | ||
| Chest pain | 0.635 | 0.225 | ||
| No | 652 | 147 | ||
| Yes | 1 | 0 | ||
| Heart failure (HF) | <0.001 a | 54.185 | ||
| No | 366 | 33 | ||
| Yes | 287 | 114 | ||
| HF with reduced ejection fraction (HFREF) | <0.001 a | 46.062 | ||
| No | 433 | 53 | ||
| Yes | 220 | 94 | ||
| HF with normal ejection fraction (HFNEF) | 0.197 | 1.662 | ||
| No | 584 | 126 | ||
| Yes | 69 | 21 | ||
| Valvular heart disease (Valvular) | 0.224 | 1.480 | ||
| No | 620 | 143 | ||
| Yes | 33 | 4 | ||
| Complete heart block (CHB) | 0.515 | 0.425 | ||
| No | 637 | 142 | ||
| Yes | 16 | 5 | ||
| Sick sinus syndrome (SSS) | 0.207 | 1.590 | ||
| No | 646 | 147 | ||
| Yes | 7 | 0 | ||
| Acute kidney injury (AKI) | <0.001 a | 23.843 | ||
| No | 442 | 68 | ||
| Yes | 211 | 79 | ||
| Cerebrovascular accident infract (CVAI) | 0.099 | 2.726 | ||
| No | 619 | 144 | ||
| Yes | 34 | 3 | ||
| CVA BLEED | 0.248 | 1.337 | ||
| No | 652 | 146 | ||
| Yes | 1 | 1 | ||
| Atrial fibrillation (AF) | 0.789 | 0.072 | ||
| No | 586 | 133 | ||
| Yes | 67 | 14 | ||
| Ventricular tachycardia (VT) | <0.001 a | 32.691 | ||
| No | 634 | 126 | ||
| Yes | 19 | 21 | ||
| PAROXYSMAL SUPRA VT (PSVT) | 0.410 | 0.678 | ||
| No | 650 | 147 | ||
| Yes | 3 | 0 | ||
| Congenital Heart disease (CONGENITAL) | 0.207 | 1.590 | ||
| No | 646 | 147 | ||
| Yes | 7 | 0 | ||
| Urinary tract infection (UTI) | <0.001 a | 17.654 | ||
| No | 573 | 146 | ||
| Yes | 80 | 1 | ||
| Neuro cardiogenic syncope (NCS) | 0.243 | 1.361 | ||
| No | 647 | 147 | ||
| Yes | 6 | 0 | ||
| Orthostatic | 0.477 | 0.506 | ||
| No | 638 | 145 | ||
| Yes | 14 | 2 | ||
| Infective endocarditis | 0.635 | 0.225 | ||
| No | 652 | 147 | ||
| Yes | 1 | 0 | ||
| Deep venous thrombosis (DVT) | 0.571 | 0.320 | ||
| No | 645 | 146 | ||
| Yes | 8 | 1 | ||
| Cardiogenic shock | <0.001 a | 198.708 | ||
| No | 617 | 74 | ||
| Yes | 36 | 73 | ||
| Shock | <0.001 a | 219.871 | ||
| No | 628 | 77 | ||
| Yes | 25 | 70 | ||
| Embolism | 0.032 a | 4.618 | ||
| No | 633 | 147 | ||
| Yes | 20 | 0 | ||
| Chest infection | 0.274 | 1.199 | ||
| No | 640 | 146 | ||
| Yes | 13 | 1 | ||
a Statistical significance at a P-value of 0.05.
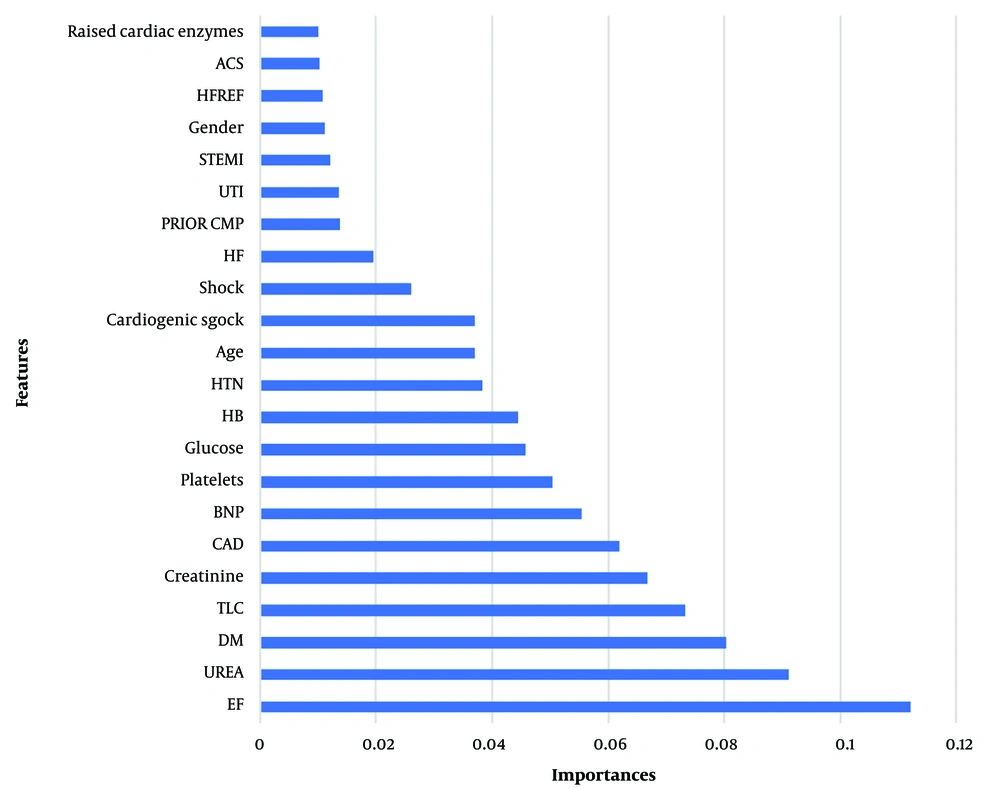
After employing the RF algorithm to identify the variables with the greatest influence on the outcome variable, the analysis revealed that ejection fraction (EF), UREA, and diabetes mellitus (DM) possessed the highest impact. Among the pool of 46 research variables, we prioritized the first 22 based on expert opinion from the field (Figure 1).
After analyzing the impact of each variable, the performance of RF and CB models was evaluated using 5-fold cross-validation. The results, presented as mean (standard deviation), indicated that CB outperformed the other model in terms of performance (Table 3).
| Models | Accuracy | Precision | Recall | F1 Score |
|---|---|---|---|---|
| RF | 0.93 ± 0.04 | 0.94 ± 0.01 | 0.92 ± 0.08 | 0.93 ± 0.04 |
| CB | 0.94 ± 0.03 | 0.94 ± 0.02 | 0.94 ± 0.06 | 0.94 ± 0.04 |
a Values are expressed as mean ± SD.
In summary, both models performed well in terms of accuracy, precision, recall, and F1 score. However, CB achieved a slightly higher recall rate (94% vs. 92%) and overall F1 score (94% vs. 93%) than the RF model.
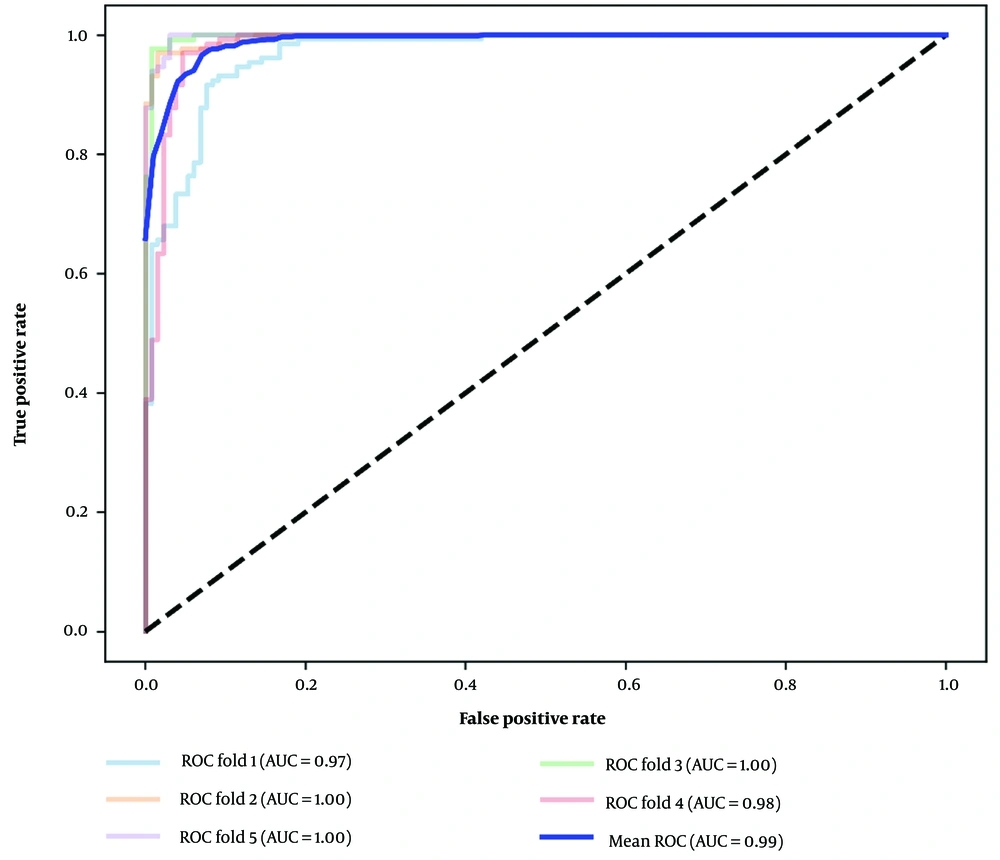
The ROC curves with k-fold cross-validation offer several advantages. It allows for a fairer comparison of model performance, as cross-validation provides more accurate estimates. Additionally, it helps assess the robustness of the model by evaluating its performance across various data subsets, providing a comprehensive understanding of performance across different distributions. The ROC curve also allows for a trade-off analysis between
5. Discussion
The primary objective of this study was to predict the likelihood of mortality among patients in the ED. To achieve this objective, ensemble models were employed, specifically chosen from the bagging models, i.e., the RF and CB models, in the boosting mode.
Based on the research findings, the CB model displayed better performance than the RF, albeit with a minor advantage. However, it is important to consider the unique dataset characteristics and project objectives when selecting between CB and RF classification. To identify the most suitable algorithm, it is advisable to conduct experiments and evaluate the performance of various algorithms on the provided dataset. Based on previous research, it has been consistently demonstrated that ML outperforms traditional scoring methods in terms of performance. Furthermore, recent studies have shown that the highest predictive accuracy achieved so far is 92%. However, upon reviewing the present study, it was observed that analyzing additional patient records and incorporating more variables can enhance the model's accuracy by up to 94%.
The study revealed significant variations in variables, including age, Total Leukocyte Count (TLC), platelet count, and urea levels, between the patients who expired and those who were discharged. Safaei et al. (24) conducted a study similar to ours, where they developed an extremely precise and effective CB model to anticipate mortality after patients were discharged from the ICU. They focused on data collected within the initial 24 hours of hospitalization. The outcomes of their research revealed a range of significant factors, such as age, heart rate, respiration rate, blood urea nitrogen, and creatinine level, which greatly impacted mortality prediction.
Furthermore, to enhance the patient's condition in the ED and ensure the effective allocation of resources, it is advised to perceive the admission and discharge of patients as a cohesive process. Utilizing simulation techniques can aid in refining this process and optimizing the distribution of resources. Hence, forthcoming research should concentrate on augmenting resource efficiency and determining the optimal allocation of resources, prioritizing patients in critical conditions.
5.1. Conclusions
This study sheds light on the exceptional accuracy and efficiency of ML in predicting ED mortality, surpassing the performance of traditional models. Implementing such models can result in significant improvements in early diagnosis and intervention. This, in turn, allows for optimal resource allocation in the ED, preventing the excessive consumption of resources and ultimately saving lives while enhancing patient outcomes.