1. Context
Soldiers are confronted with a variety of vector-borne threats and diseases during their missions in war areas. Among all threats, malaria holds an important place as a major health problem in tropical or hyperendemic areas. Malaria control in war areas has been deemed one of the most challenging goals of different armies throughout history. According to the world health organization (WHO), the integrated vector management (IVM) is a promising method to improve the efficacy, cost-effectiveness, ecological acceptability, and sustainability of malaria control. Malaria is a parasitic infection induced by a Plasmodium, which lives part of its life in Anopheles mosquitoes and part in humans (1) and creates one of the most important health concerns on a global scale insofar as over one-third of the world’s population is threatened by malaria. It prevails in the tropical areas of South America, Central America, Africa, and Asia and endangers millions of the inhabitants of endemic areas. The annual malaria occurrence rates are estimated at about 350 to 500 million worldwide. In 2009, the number of the infected victims was estimated at about 225 million and the number of deaths reached 781000 (2). A comprehension of the current situation of malaria requires a review of the history of this disease and the preceding global attempts at its elimination and control. In the mid-19th century, malaria was almost prevalent in many territories and countries of the world, with the adverse impacts of the disease affecting approximately 90% of the world’s population, such that it was observed even in the proximity of the Arctic Circle (3). Charles Laveran, a French military doctor in the army bases in Algeria during 1880s, was the first scientist to identify the Protozoa as the cause of the parasitic disease of malaria. Indeed, the Plasmodium parasite inside the red blood cells was first observed by Laveran. Later on in 1898, Sir Ronald Ross, a physician in the British army in India, proved that mosquitoes are principally responsible for malaria transmission and that the malaria insect belongs to the genus Anopheles (4). Throughout history, although armies have tried different methods to fight malaria, it still remains as a military medical challenge because the implementation of conventional vector-control methods has led to unsustainable results. The failures of conventional methods to eradicate malaria have obvious reasons, including insecticide resistance, which is observed in Anopheles mosquitoes, and undesirable environmental and health impacts caused by the excessive application of chemical insecticides. In contrast, the IVM methods have achieved significant victories in the battle against malaria and acknowledgement by the WHO.
1.1. History of Malaria
From ancient times, malaria was known to man. Different ancient records from 2700 BC, including the Chinese Canon of Medicine or Nei Ching, mentioned malaria indications and associated enlarged spleens with fevers. Also in 1550 BC, the Ebers Papyrus discussed inconsistent splenomegaly, fevers, and rigors and introduced the extracted oil of the Balantines tree as an anti-mosquito concentration. The first scientist to establish a connection between the distance of stagnant water bodies and the occurrence of fevers in the local population was Hippocrates from ancient Egypt. Romans, too, linked fever incidence with marshes and pioneered to fill marshlands and swamps. Malaria was among the most important causes of the sickness of troops at some stages in the Second World War in endemic areas, including tropical and Mediterranean zones. The occurrence of the disease in its transmission season period endangered numerous military campaigns and operations (5). For example, the malaria incidence in 1943 was as high as 746/1000 in the Indo-Burma Front. Even in the Middle East, the admission rate for malaria rose to a peak of 677 in 1940 and fell to 380/1000 in 1945. The infection rates in West Africa were reported as follows: 762/1000 in 1942; 442/1000 in 1943; 278/1000 in 1944; and 92/1000 in 1945. In Europe, the disease was epidemic at a steady annual rate of 13.8/1000 between July and September 1944, particularly in the northern and western parts of the continent, but this rate fell to 9/1000 in October and December 1944 (6). In the meantime, in the Central Mediterranean Zone and the northern part of Africa, malaria had a much higher occurrence rate and hunted down the troops. These reported data should, however, be interpreted with caution because the ethnic compositions of the troops were different (7). The Eighth Army of the British forces encountered an exceptionally harsh condition of the disease in Sicily in the summer of 1943 just before the Italian mainland invasion. Nearly 8000 soldiers were infected with malaria in 1944 before the Battle for Cassino, when passing from the Roman Campagna in Monte San Biagio (8). In order to fight malaria, the anti-malarial unit (AMU) was established. The usual designation to an armed forces division was 6 AMUs, which included 3 permanent service units and 1 was joined to every division. Another form of malaria unit was devised in the Indian subcontinent and was known as the malaria forward treatment (MFTU), which made it possible to manage severe cases in war fronts. The MFTU consisted of small units which provided 200 hospital beds. In 1945, it was reported that a successful cure of malaria infection usually required about 8 - 9 days (9). Anti-malarial divisions, staffed by experienced entomologists, were vitally important and their investigation into the bionomics of Anopheles was of great value. The Indo-Burma Front and Ceylon (Sri Lanka) showed the difference in the epidemiological characteristics of Anopheles. Between 6 and 9 months after the first infection, a relapse of Plasmodium vivax (P. vivax) malaria caused serious issues. Because half of the relapses were observed within the first 3 months, it was difficult to conclude whether a particular relapse was a true relapse or a fresh infection. Malaria also became a grave concern in the United States Armed Forces in many parts of the world (10). Almost 500000 cases of malaria infection were reported in a 4-year period, with the peak rate in 1943 - 1944 (69000 cases). Also, based on the reports, nearly 9 million man-days were lost in the United States Armed Forces (11). Owing to the high infection incidence in the military campaign near Sicily, the British Eighth and the United States Seventh Armies lost the battle: there were more losses due to malaria than there were battle injuries; the United States Seventh Army recorded 9892 victims of malaria, while the battle casualties were only 8375 individuals (12). Hereupon, the malaria control in war areas (MCWA) was founded during 1942 - 1945 to control malaria near the military training bases in the southern parts of the United States and its territorial lands, especially in the areas where malaria was a serious problem. Most of the military bases were located in areas where mosquitoes were abundant. The MCWA aimed to prevent the reintroduction of malaria into the civilian population by mosquitoes that would have fed on malaria-infected warriors when they were returning from the fronts or training camps located in malaria endemic areas. The United States Armed Forces were ordered by the Congress in 1982 to lead investigations for infectious diseases. The significance of vaccines and medical solutions was emphasized in Executive Order 13139, issued in 1999, expressing that It is the policy of the United States Government to provide our military personnel with safe and effective vaccines, antidotes, and treatments… (13). Due to the extensive malaria incidence, the war in the Korean Peninsula and Southeast Asia ended in failure. The occurrence rate of malaria infection among the Viet Cong units was reported between 50% and 75%. So severe was the incidence of the disease that it resulted in raids on dispensaries and plantations to access supplies of drugs. In Vietnam (1965) around 1070 of the United States Armed Forces were infected for every day they spent in the war area. In 1966, some infantry units had a malaria incidence rate of 30% of the strength per month (14).
2. Objectives
We seek to introduce the IVM with a view to translating its environmental strategies into military applications so as to affect sustainable control of malaria in war areas.
3. Data Sources
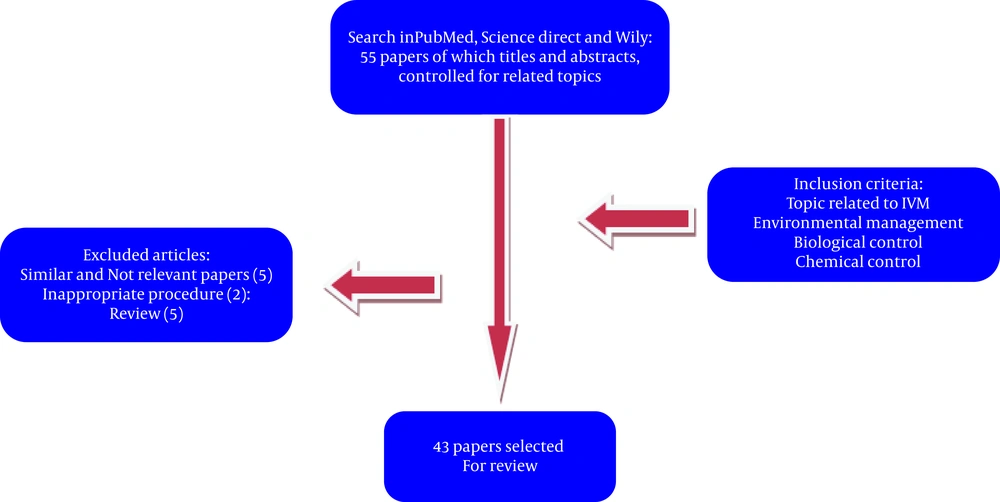
The searching process for retrieving relevant publications was conducted in PubMed, Wiley, and ScienceDirect for all studies until November 10th, 2014. The principal search of the papers was carried out by one author (Milad. D) under the supervision of two investigators (Farshad. N and Amir. N), who are expert in medical sciences. The following keywords (MeSH terms) were used: malaria control; malaria prevention; and war areas. No restrictions on paper status or publication date were exerted; hence, different language papers were obtained, but only English language papers were included. Additionally, the reference lists of the relevant papers were reviewed for other appropriate scientific sources. As vectors, the mosquito species Anopheles were considered as the main subject. In order to evaluate the future status of the IVM strategies in military medicine as an environmental approach, relevant studies in the field of biological control, chemical control, and environmental management related to malaria were retrieved. Thirty papers were obtained from ScienceDirect and 25 from Wiley online. Unrelated and similar papers were removed from the review process, and 50 publications remained. Thereafter, according to the inclusion/exclusion criteria decided by the authors, 43 papers were selected for the final review. In this study, the inclusion criteria were defined and specified in terms of the IVM methods, including environmental management, biological control plans, and chemical control techniques.
4. Study Selection
The selected publications were expected to discuss topics vis-à-vis the IVM such as chemical and biological control methods and were required to report on the IVM outcomes. The present review article only included (i) research papers, (ii) reports on the IVM, and (iii) comparative studies between the IVM and the conventional methods.
5. Data Extraction
The information and content from the retrieved studies were reviewed based on topics concerning the IVM studies and recommendations.
6. Results
From 43 publications finalized for the inclusion in the review process, some articles were about historical perspective on malariology and malaria in war time, some discussed the IVM, and some proposed new biological and chemical control methods for malaria prevention and treatment in endemic areas. The outcomes from the reviewed studies were investigated and categorized by the authors in five sub-categories, namely transmission, symptoms and diagnosis, biological control, chemical control, and environmental management. Based on the reviewed publications, the IVM is an environmental friendly, sustainable, and cost-effective method for malaria prevention and control in endemic areas, including tropical and subtropical areas, marshy lands, and humid dense forests, which motivated us to assess the IVM concepts in relation to war areas from a military perspective. Fortunately, many promising and successful results in the utilization of the IVM in hyperendemic areas have been observed and documented. One of the most appealing applications of the IVM is its capability to confer preventive advantages by habitat modifications, especially in war areas, which may lead to cost-effective malaria treatment. The IVM was acknowledged and promoted by the WHO after its interesting results in African and Asian endemic countries. The IVM strategies can protect soldiers in endemic areas by manipulating the concept of prevention through the reduction and interruption of the transmission cycle of malaria. Different types of malaria vector-control methods have been proposed by experts such as using insecticide-treated nets, long-lasting treated nets, and indoor residual spraying (15). The above methods are classified under the chemical control plans and should be combined with other environmental techniques for effective adult and larval control of malaria mosquitoes. According to the recommendations of the WHO, the use of proper combinations of chemical and nonchemical methods can guarantee the successful implementation of the IVM. The IVM strategies are practical and effective inasmuch as they offer a set of options of vector-control methods that can be applied in different combinations for multiple geological and ecological conditions. The AMUs should seek to target vectors in every stage of their life cycle such as larval and pupal stage in their breeding habitats like swamps and marshlands.
6.1. Transmission
In static base units, the degree of the potential exposure to malaria depends on local epidemiological conditions such as closeness to large mosquito-breeding areas and type of military habitations. The dynamic relation between parasite, men, vector and environment indicates the transmission rate of malaria (16). Malaria transmission through contaminated blood or the infection of a fetus by its mother during pregnancy is really rare (17, 18). Many biological and environmental factors shape the transmission of malaria in a given location. An epidemic may occur when conditions such as climate effects along with human intervention allow the mosquito population to reach high figures. The incidence of malaria is mainly dependent on the environmental factors that control mosquito survival and breeding (19, 20). The IVM combines chemical and non-chemical vector-control techniques with other disease-controlling parameters such as local vector biology and the dynamics that influence the disease transmission and morbidity.
6.2. Symptoms and Diagnosis
Malaria can be diagnosed by a chain of continuous attacks, or paroxysms, including three phases: the chills phase; fever phase; and sweating phase. In addition to chills, the patient is likely to have malaise, headache, fatigue, and muscular pains, accompanied by inconsistent nausea, diarrhea, and vomiting. Simply in less than 2 hours, the body temperature rises, the patient feels hotness and dryness on his skin, and finally, when the body temperature falls, a drench sweat begins and the patient feels exhausted and sleepy. The symptoms first appear about 10 to 16 days after the infectious mosquito bite. When the red blood cells become infected and break simultaneously, malaria attacks recur every 2 days for P. ovale malaria and P.vivax and every 3 days for P. malariae at regular periods (21, 22). In case of the first malaria incidence, health care units should react rapidly and treat the patients. The identification of the Anopheles species present in particular geographical areas where the soldiers become infected is essential to indicate the proper prescriptions. Before deploying the troops to an area with malaria risk, an expert in health care should try to acquire comprehensive information on what medications to carry, during and after the mission. The conditions of the mission, destination, and types of activities that the soldier will undertake would determine the health risks for malaria infection. For instance, a combatant who settles even one night in an endemic area becomes exposed to infection. A practical manual of information on how to limit contact with mosquitoes and updated guidelines on anti-malarial drugs should be prepared before the deployment of troops (23). New strategies for malaria prevention and control concentrate on the IVM and support a relation between health and environment (24). The IVM is a dynamic method which discusses specific strategies to achieve disease control in the most cost-effective manner while minimizing negative impacts on ecosystems, reducing the adverse health effects of insecticides and their toxic residues, and decreasing the insect resistance to some extensively applied drugs and pesticides (25, 26). Compared with a conventional method of vector control (e.g. chemical spraying), the IVM highlights the value of understanding the local patterns and the ecology of the local vector for disease distribution and decides the correct vector-control methods from the range of available options (27, 28). The IVM also proposes a well-thought-out structure for the protection of the individual that employs environmental management tools like physical barriers along with chemicals such as insecticide-treated nets. And last but not least, it should be borne in mind that the most important purpose of treatment is to provide access to appropriate medication for the initial infection stages to avoid the development of the life-threatening stages, which require hospital admission. Nevertheless, providing perfect treatment such as chemotherapy is still problematic, particularly in war areas (29, 30).
6.3. Biological Control
The WHO published a special issue in 2004 to declare the Global Strategic Framework for Integrated Vector Management, which clarifies the purposes, requirements, and principles of the IVM methods (31). The IVM is supported and optimized by the WHO as a rational decision-making procedure for the inclusive exploitation of resources for vector prevention and control (32, 33). The exploitation of biological toxins for targeted mosquito vectors and the employment of natural enemies to reach efficient disease control are the principles of the biological control (13, 34). Alternative examples in this method include Larvivorous fish, invertebrate predators, nematodes, Protozoa, fungi, and bacteria.
Biological vector control is an innovative alternative in malaria control, when a lack of reliable vaccines and the emergence of drug resistance and unaffordable anti-malarial medications result in the failure in malaria control (35). In order to control malaria in war areas efficiently, the following steps are recommended: appropriate supervision of the IVM; identification of innovative and environment-friendly alternative technologies; exploration and development of logical compatible interventions in military bases; promotion of the existing strategies regarding their effectiveness and efficiency and their combinations with biological control of vectors; and endorsement of environmental management and surveillance (36).
6.4. Chemical Control
Chemicals and insecticides are integral parts of the IVM and play a key role in malaria control scenarios. They should, however, be launched swiftly to be effective at relatively low costs. Accurate timing in chemical application is compulsory at the early stages in the control process because it allows further control methods to be established and developed in an integrated vector-control strategy (37, 38). Chemical application methods comprise targeted residual spraying, larviciding, and space spraying.
In order to have an ecofriendly formulation of insecticides, the active or technical material should be combined with a variety of components that pose no toxic effects to environment as well as human. With respect to mud walls, because of the absorbent nature of their surfaces, the spraying process should be conducted in a way that the chemical agent particles remain on the surface and not be absorbed into the walls given the porosity of their surface (39).
6.5. Environmental Management
In case of the long-term settlement of troops, environmental management strategies should be employed to decrease the malaria infection burden through sustainable approaches. Environmental management strategies emphasize the avoidance of the formation of vector-breeding sites by altering natural habitats and improving soldiers’ habitation to lessen the abundance of the vectors (40, 41). Examples of such strategies include marsh alteration, filling, grading, draining, vegetative plantings, and house screening (42).
7. Conclusions
The lessons of malaria in the Second World War resulted in a successful prevention of the disease in the United States Armed Forces in several fronts around the world. The salient points in this success story are directives, organization, cooperation, integration, training, and skill. In particular, awareness about malaria in the level of the troops and in the high command is necessary. In 1945, malaria was successfully controlled with dichlorodiphenyltrichloroethane (DDT). The global malaria eradication campaign was approved and launched by the 8th world health assembly in 1955 (15). This campaign was also launched to control and prevent the disease from war areas at the peak of malaria infection. Malaria control via the IVM depends on the presence of anti-malarial trained units. Health care units in tropical malarious war areas should develop an infrastructure capable of controlling malaria for the short-term and long-term settlement of troops. To control malaria under the critical conditions of war areas, an appropriate immediate response is expected from the trained units in the event of the first malaria observation. AMUs should form, train, investigate the epidemiological situation, and recommend control measures. Furthermore, AMUs should work in lockstep with field hygiene divisions and the number of AMUs should be determined based on the available resources (29, 30). The members of AMUs should be knowledgeable about the following stages: geographical reconnaissance; attack phase (vector control until the parasite rate is low); and maintenance phase (passive case detection and effective treatment).
In military base camps, the application of collective methods of prevention via insecticides, larvicides, or environmental management for mosquito control with special emphasis on prevention should be launched immediately and monitored regularly.
Unfortunately, the IVM has not been introduced to and practiced by armies in most countries. This shortcoming can be explained by a lack of environmental concerns and sustainable policies to guide the integrated approach in these countries. Nonetheless, in our view, the IVM should be recognized as a set of well-thought-out methods for the control and prevention of vector-borne diseases. The present paper sought to endorse the IVM and translate its strategies into practical actions for the armed forces. In order to improve the equipment in entomology and vector-control section, the promotion of environmental knowledge to guarantee a sustainable and ecofriendly control method seems crucial. The mismanagement of insecticides may cause vector resistance to them, especially to pyrethroids, which would bring about a catastrophe. Armies should create a policy framework and try to practice the IVM techniques with military insight before they can establish an IVM monitoring section and conduct regular development procedures. The extensive use of insecticide-treated bed nets should become compulsory for soldiers, not least in endemic areas. A successful operation necessitates that a malaria-risk map of the area be produced by reliable remote-sensing and GIS tools accurately. During the last decade, the implementation of the IVM has resulted in malaria control in endemic areas. Therefore, employing the IVM methods in war areas and military bases located in high-risk regions looks promising, provided that sanitary units become familiar with the environmental concepts of the IVM. However, one of the remaining concerns is the climate change, which may render some areas of the world that are not currently endemic to malaria more climatically favored to the transmission of the disease. In the malaria control process in war areas, prevention and treatment should be implemented simultaneously. Even the prevention of the disease transmission should be triggered in the larval source. Only in this situation can a significant reduction in malaria infection rates be expected. We strongly believe that international cooperation, followed by early diagnosis and prompt treatment, is fundamental to the successful application of the IVM to malaria prevention and control.