1. Background
Oxidative stress is a state of imbalance in capacity to neutralize the free radicals and its production. Depletion of endogenous antioxidant reserves and excessive reactive oxygen species (ROS) production can induce oxidative stress (1). Free radicals can activate the transcription factor κB or nuclear protein κB (NF-κB) by stimulating metabolic pathways which, in turn, increases the expression of pro-inflammatory cytokines (2). In patients with cardiovascular disease inflammatory markers such as tumor necrosis factor-α (TNF-α), high-sensitivity C-reactive protein (hs-CRP), interleukin-1 (IL-1), IL-6, IL-18, and FAS ligand are high (3). Cigarette smoking, ingestion of non-steroidal anti-inflammatory drugs (NSAIDs), alcohol consumption, ultraviolet (UV) radiation, and many other exogenous agents can result in ROS production. Also, elevated levels of ROS can be due to various inflammatory processes, ischemia-reperfusion (I/R) injury, and infections (4). It has been shown that gamma-glutamylcysteine synthetase (GCS), glutathione peroxidase (GPX), and mitochondrial superoxide dismutase (MnSOD) upregulated as well as oxidative damage reduced in rats exposed to exercise (chronic training) (5). It seems that all adaptation to long-term trainings occur after individual training sessions (6), nevertheless its mechanisms are not still clear. In this regard, eight weeks (five days a week) of high-intensity interval training (HIIT) significantly increased SOD and CAT in the heart tissue of diabetic rats (7). One single session and 12 weeks of swim training significantly decreased serum of hs-CRP and TNF-α in type 2 diabetic rats (8). Ten weeks of intensive interval exercise had no significant effect on intercellular adhesion molecule-1 (ICAM-1), MCP-1, and serum IL-10 in overweight women (9). Four weeks, five times per week, of aerobic exercise training had no significant effect on TNF-α and NF-κB in the lung tissue of male rats (10). Fourteen weeks of exercise training had a significant effect on decreased caspase-3 and NF-κB in the acute pressure overload of male rats (11). Medicinal plants have long been used as a therapeutic approach (12). On one hand, due to the tendency of different humans around the world to the use of medicinal herbs and the therapeutic features of some plants, and on the other hand, due to the reduction of the complications of chemical drugs, the consumption of herbs is increasing (13).
Portulaca oleracea is from the Portulacaceae family and is commonly known as purslane, and commonly used as therapeutic plants. P. oleracea is including antioxidants like β-carotene, vitamins C, vitamin E, and vitamin A (14). It is reported that the purslane extract is a factor in improving lipid profile and body weight in diabetic rats (15). In addition, eight weeks of resistance training and aerobic exercise training simultaneously with consumption of purslane seeds by women with type 2 diabetes improved pro-oxidant and antioxidant balance. It also prevented exercise-induced oxidative stress (16), and also had a significant effect on the decrease in NF-κB and CRP (17). A total of 200 g/kg of purslane seed oil consumption for 21 consecutive days significantly increase GP, SOD, and GSH as well as decreased ALP, AST, and LDH in female mice.
2. Objectives
Studies have recently shown that the simultaneous combination of medicinal plants such as the Portulacaceae family and exercise training has beneficial effects on some of the diseases, such as diabetes (3, 18-20). However, the review of literature shows that these studies have not specifically addressed the NF-κB and CRP, especially in cardiovascular disease. On the other hand, the anti-oxidative effects of different doses of purslane seed have been reported in some recent studies (21, 22), however, it seems that the effect of the most appropriate dosage of purslane seed is still unclear; therefore, the present study,aims to examine the interactive effects of endurance training and the purslane seed after administration of the appropriate dosage of this medicinal plant on NF-κB and CRP in the heart tissue of rats poisoned by H2O2.
3. Methods
In this experimental study, 64 adult Sprague Dawley male rats (weight = 180 - 200 g) were purchased from the animal breeding center located in the house of animals of Tehran Pasteur Institute in 2017. All rats were held at the standard physiological laboratory (free access to water, environmental temperature 2 ± 22°C, controlled cycle of 12-hour light and 12-hour dark) and special diets of rats. Following seven days of adaptability, they were randomly assigned into eight groups of eight, including: (1) control, (2) endurance training (ET), (3) endurance training with 50 mg/kg/day supplementation of purslane seed (ET + P50), (4) endurance training with 200 mg/kg/day supplementation of purslane seed (ET + P200), (5) endurance training with 400 mg/kg/day supplementation of purslane seed (ET + P400), (6) 50 mg/kg/day supplementation of purslane seed (PS50), (7) 200 mg/kg/day supplementation of purslane seed (PS200), and (8) 400 mg/kg/day supplementation of purslane seed (PS 400). During eight weeks groups one through eight received 1 mmol/kg H2O2 three times per week intraperitoneally (22); groups two through four ran on the treadmill three sessions per week, and groups two through eight received purslane seed intraperitoneally daily (22).
3.1. Purslane Seeds Preparation
Purslane seeds were washed then air dried for seven days (at a room temperature). Purslane seeds powdered and dissolved in the distilled water. In the present study, according to the method of Soori et al. (22) with voucher specimen No.15-04979, plant identification was determined.
3.2. Training Protocol
After one week of acclimatization, all rats in the training groups ran on a rodent treadmill for eight weeks and performed endurance trainings. In the first week, duration of the training gradually increased from 30 minutes of running with intensity of eight m/minute for 30 minutes with an intensity of 12 m/minute in the second week while the 10-degree gradient of the treadmill was steady, in the third week 45 minutes with intensity of 16 m/minute, and in the fourth week 45 minutes with intensity of 20 m/minute. In addition, during the fifth to eighth weeks, the speed remained fixed at 60 minutes with an intensity of 20 m/min (23).
3.3. Tissue Sampling and Determination of Oxidative Stress Markers
A total of 48 hours after the last training session, all rats were anesthetized by xylazine and ketamine, then by cervical dislocation sacrificed. The heart tissue was removed and prior to protein extraction, snap-frozen in the liquid nitrogen. Commercial NF-κB and CRP ELISA kits (Cusabio; USA) were used for measurement of NF-κB and CRP.
3.4. Statistical Analysis
Shapiro-Wilk, two-way ANOVA with the Bonferroni post hoc tests were used for statistical analysis of data in SPSS software (version 20) (P ≤ 0.05).
4. Results
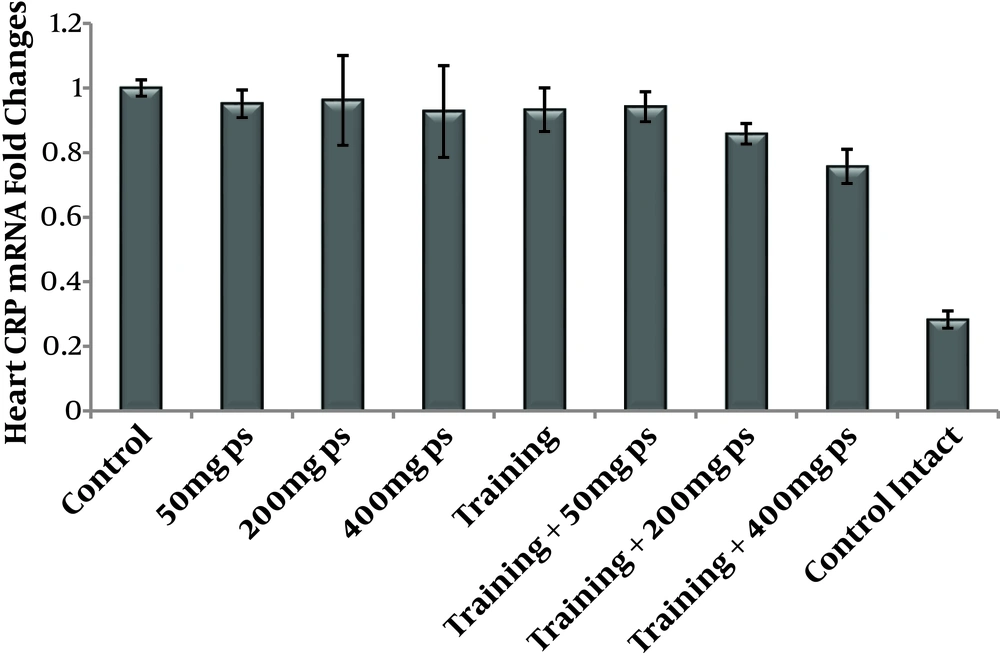
The results of the Shapiro-Wilk test in Table 1 showed that the data have normal distribution in the research groups. The protein levels of expression of NF-κB and CRP in the heart tissue of rats are presented in Table 2. Two-way ANOVA test showed that endurance training significantly increased CRP protein levels (F = 15.17, P = 0.001, µ = 0.487), however, the purslane seed consumption significantly reduced CRP protein levels (Table 3). It should be noted that the results of the Bonferroni post hoc test in Table 4 showed that this reduction was dose-dependent; indeed, with an increase in the dose of purslane seed, the levels of CRP protein reduced (F = 28.11, P = 0.0.001, µ = 0.841); also, endurance training with purslane seed extract consumption had interactive effects on reduction of CRP protein levels (F = 39.92, P = 0.0.001, µ = 0.897).
| Variables and Group | Shapiro-Wilk | |
|---|---|---|
| Statistic | P Value | |
| CRP | ||
| Control | 0.985 | 0.762 |
| 50 mg ps | 0.809 | 0.136 |
| 200 mg ps | 0.871 | 0.297 |
| 400 mg ps | 0.998 | 0.923 |
| Training | 0.813 | 0.146 |
| Training + 50 mg ps | 0.998 | 0.905 |
| Training + 200 mg ps | 0.955 | 0.590 |
| Training + 400 mg ps | 1.000 | 0.962 |
| Control intact | 0.971 | 0.675 |
| NF-κB | ||
| Control | 0.999 | 0.942 |
| 50 mg ps | 0.987 | 0.778 |
| 200 mg ps | 0.898 | 0.380 |
| 400 mg ps | 0.856 | 0.256 |
| Training | 0.880 | 0.324 |
| Training + 50 mg ps | 0.995 | 0.860 |
| Training + 200 mg ps | 0.909 | 0.415 |
| Training + 400 mg ps | 0.943 | 0.541 |
| Control intact | 0.999 | 0.946 |
| CRP | NF-κB | |
|---|---|---|
| Control | 1.00 ± 0.025 | 1.00 ± 0.013 |
| 50 mg/kg purslane seed | 0.951 ± 0.042 | 1.047 ± 0.105 |
| 200 mg/kg purslane seed | 0.961 ± 0.138 | 1.048 ± 0.031 |
| 400 mg/kg purslane seed | 0.927 ± 0.142 | 0.962 ± 0.111 |
| Endurance training | 0.932 ± 0.067 | 0.945 ± 0.030 |
| Endurance training with 50 mg/kg purslane seed | 0.941 ± 0.046 | 0.911 ± 0.063 |
| Endurance training with 200 mg/kg purslane seed | 0.858 ± 0.031 | 0.826 ± 0.062 |
| Endurance training with 400 mg/kg purslane seed | 0.757 ± 0.052 | 0.837 ± 0.069 |
| Variables and Factor | Sum of Square | Df | Means of Square | F | P Value | Effect Size |
|---|---|---|---|---|---|---|
| CRP | ||||||
| Endurance training | 0.149 | 1 | 0.149 | 15.17 | 0.001 | 0.48 |
| Purslane seed | 0.831 | 3 | 0.277 | 28.11 | 0.0001 | 0.84 |
| Purslane seed and endurance training | 1.15 | 3 | 0.383 | 38.92 | 0.0001 | 0.87 |
| NF-κB | ||||||
| Endurance training | 0.133 | 1 | 0.133 | 31.90 | 0.001 | 0.66 |
| Purslane seed | 0.919 | 3 | 0.306 | 73.61 | 0.001 | 0.93 |
| Purslane seed and endurance training | 1.08 | 3 | 0.361 | 86.82 | 0.001 | 0.94 |
| Group | Mean Difference | P Value |
|---|---|---|
| NF-κB | ||
| 50 mg/kg | ||
| 200 mg/kg | 0.092 | 0.154 |
| 400 mg/kg | 0.180 | 0.001 |
| Control | 0.518 | 0.001 |
| 200 mg/kg | ||
| 400 mg/kg | 0.088 | 0.186 |
| Control | 0.426 | 0.001 |
| Control | 0.338 | 0.001 |
| CRP | ||
| 50 mg/kg | ||
| 200 mg/kg | 0.039 | 0.99 |
| 400 mg/kg | 0.104 | 0.52 |
| Control | 0.469 | 0.001 |
| 200 mg/kg | ||
| 400 mg/kg | 0.066 | 0.99 |
| Control | 0.430 | 0.001 |
| Control | 0.364 | 0.001 |
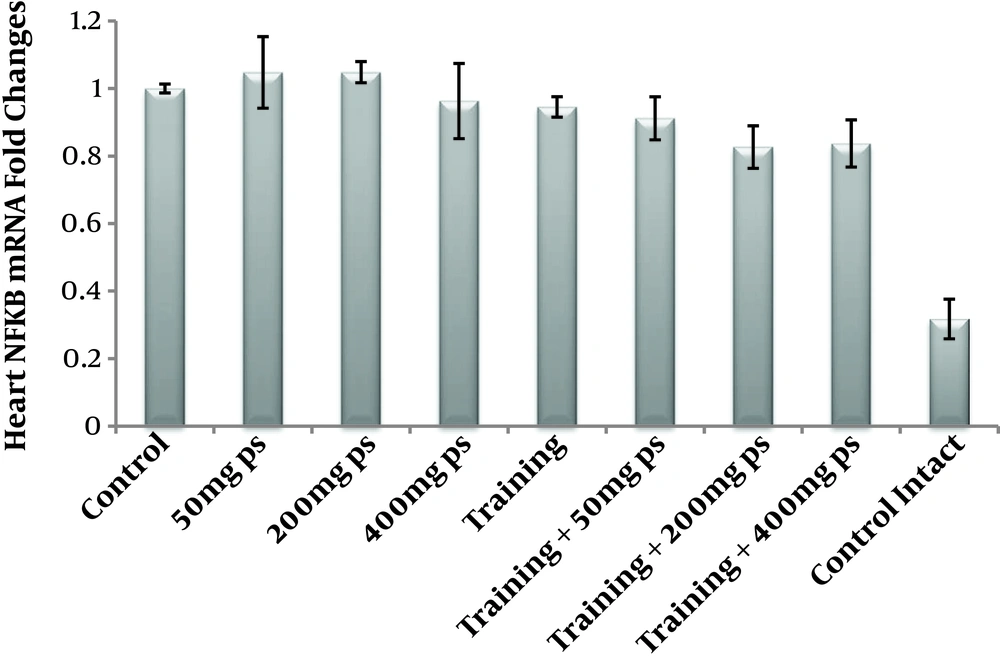
Two-way ANOVA test showed that endurance training had no significant effect on NF-κB protein levels (F = 2.04, P = 0.172, µ = 0.113), however, the purslane seed consumption significantly reduced NF-κB protein levels (Table 3). It should be noted that the results of the Bonferroni post hoc test in Table 4 showed that this reduction was dose-dependent; indeed, with an increase in the dose of purslane seed, the levels of NF-κB protein levels reduced (F = 12.41, P = 0.0.001, µ = 0.699); in addition, endurance training with purslane seed consumption had interactive effects on the reduction of NF-κB protein levels (F = 17.32, P = 0.0.001, µ = 0.765) Figures 1 and 2.
5. Discussion
In the present study, endurance training significantly increased CRP protein levels, and had no significant effect on NF-κB protein levels. Researchers reported that the severity of heart failure (HF) can be due to increased levels of oxidative stress markers. Furthermore, the researchers have demonstrated the relationship between the severity of oxidative stress, renal function, and levels of hs-CRP and pro-B-type natriuretic peptide (pro-BNP) in patients with HF (20). All tissues can produce the free radicals by several mechanisms. Angiotensin II, growth factors, and pro-inflammatory cytokines can contribute in ROS formation (2). Also, activation of transcription factors such as DNA damage-inducible protein 153 (GADD153), NF-κB, and growth arrest increased the expression of several genes, and the p38 mitogen-activated protein kinase (MAPK) pathway can be triggered by CRP (24, 25). It seems that inside the mitochondria, several functional enzymes are susceptible to ROS-mediated damage and induce cardiac dysfunction. Increased levels of ROS by promoting mitochondrial uncoupling affects mitochondrial ETC system. Indeed, increased levels of ROS decreased mitochondrial complex III activity and reduced ATP/ADP ratio. In addition, ROS can oxidize proteins and lipids as well as intracellular molecules (like NO) which damage the cell (26). As oxygen consumption in active muscles during exercise is up to 100 times higher than at rest; the primary source of free radical and ROS production was hypothesized to be the mitochondria (27). Regular exercise by increasing antioxidant levels may affect exercise-induced oxidative stress. However, the significance of the effect of exercise on reducing CRP and NF-κB protein in the present study can be explained by increasing the H2O2 and the ineffectiveness of the antioxidant system. The researchers showed that in male rats resistance training had no significant effect on the levels of malondialdehyde (MDA) (28); 12 weeks, six days a week, and two hours of training sessions on ergometer did not significantly change the expression of SOD in lymphocytes in boys aged 15 - 16 years (29). NF-κB expression in vivo following a high-fat diet (HFD) and exercise increased; also, there was a significant correlation between NF-κB activation and weight in HFD mice. P65 expression was identical in both groups and in all tissues (30). In contrast with this, the researchers concluded that the level of CRP reduced following two weeks of aerobic training (31). In diabetic db/db mice moderate intensity trainings for eight weeks significantly reduced mRNA levels of TNFα, IL- 6, and F4/80 (macrophage marker) as well as reduced activation of IκBα/NF-κB pathway (32). Twenty weeks of standardized exercise training program had a significant effect on reduced CRP in 652 sedentary healthy white and black men and women (33). The reasons for inconsistency of the results can be the sameness in the population as well as duration and intensity of exercise. In the elderly and young people regular pedaling on ergometer bike for 12 months significantly increased glutathione reductase (GR), GPX ,and SOD as well as in young people the antioxidant increase was significantly higher than the elderly people (34).
In the present study, purslane seed consumption had a significant effect on the reduction of CRP and NF-κB protein levels and this reduction was dose-dependent of the extract; indeed, with an increase in the dose of purslane seed, the levels of CRP protein levels reduced. In the present study, the synergistic effect of various compounds found in purslane seeds can be the reason for reduction in CRP (17). In this regard, 16 weeks of consumption of purslane seed (2.5 g lunch and 5 g dinner) significantly decreased the mRNA and protein levels of TIMP-1, CRP, CST3, NF-κB, CTSS, MMP2, and nine compared to pre-experimental or the placebo group (35). A total of 100 mg/kg and 200 mg/kg purslane significantly reduced the NFκBp65, p-NFκBp65, IκBα, p-IκBα, IL-6, IL-1β, and TNFα in diabetic mice (35). Studies on the antioxidant effects of purslane seed were limited, therefore, we failed to find a study that is inconsistent with the present study; however, positive effects of 100 mg/kg/day and 400 mg/kg/day purslane seed consumption in the heart tissue of Wistar rats with levothyroxine-induced thyrotoxicosis have been reported (36). Researchers believe that the polysaccharides in purslane seed is able to clear anion superoxide, 1-1 diphenyl-2-pyridine hydrazine (DPPH), nitric oxide and hydroxyl radicals, and so it has the protection against the property free radicals. Thus, the phenolic alkaloid of this plant has an open protective effect on hydrogen peroxide, which in turn increases the lipid peroxidase (37).
Also, the present study showed that purslane seed consumption simultaneously with endurance training have interactive effects on the reduction of NF-κB and CRP and protein levels. Decrease of free fatty acids can be the season of improvements in the inflammation observed in the subjects after endurance training or purslane seed consumption or both, which deteriorate improvement of metabolic mechanism and excess of lipid availability. It is reported that endurance training and purslane seed consumption can reduce the cholesterol levels (17), which is associated with the levels of ROS and inflammation in addition to regulation of extracellular matrix (ECM). Cathepsin S (CTSS) and matrix metalloproteinase (MMPs) are proteases, which degrade at least one component ECM and contribute to inflammation and tissue remodeling. Higher levels of CTSS17 is associated with increased levels of IL-6 and CRP. balance between the protease enzymes (MMPs and CTSS) and their inhibitors (metallopeptidase inhibitor 1; TIMP-1 and CST3) are deregulated on pathological conditions such as diabetes (38).
Understanding the factor that can simultaneously modulate the effects of oxidative stress with exercise can be valuable and prevent over-loss of the cell. By activating sensitive intracellular dual pathways, reactive oxygen species increase the expression of antioxidant enzymes and other cellular protein carriers to adapt to oxidative stress and to maintain the hemostasis of the cell. High concentrations or lack of reactive oxygen species play an inhibitory role in the intracellular adaptations. As previously mentioned, purslane seed consumption and endurance training may inhibit the activation of reactive oxygen species by reducing the lipid peroxidation and thereby inhibit the remodeling and inflammation (27, 28, 34, 37). There are limited studies on the interactive effects of exercise and purslane seed extract on CRP and NF-κB. However, there have been studies that, similar to the present study, have more positive effects on these two intervention. For example, 16 weeks of aerobic training and purslane seeds consumption had an interactive effects on reducing uric acid, CRP, cholesterol, triglycerides (TG), fasting blood glucose, creatinine, urea, low-density lipoprotein (LDL), NF-κB, TIMP-1, CST3, GLP1, GLP1R, MMP9, and MMP2 as well as increasing high-density lipoprotein (HDL); eight weeks, three sessions per week of resistance training with purslane seed extract had an interactive effects on improving liver enzymes in type 2 diabetes women (37). Based on the results of this study on the inflammation of effects of sport in rats exposed to oxidative damage induced by H2O2, it is suggested that in the future studies, in a similar protocol with the present study, exercise should last for a longer time. It is also suggested that in the future studies, the levels of antioxidants and stress oxidative should also be studied in a similar way. Moreover, it is recommended that in the future studies, considering the impacts of resistance and swimming training on the heart tissue, the effects of resistance or swimming trainings and the simultaneous use of purslane seed consumption on apoptotic, inflammation indices in the rats exposed to oxidative damage induced by H2O2 should be investigated.
5.1. Conclusions
It seems that purslane seed consumption, simultaneously with endurance training, have interactive effects on the reduction of NF-κB and CRP in the heart tissue of rats poisoned by H2O2.