1. Background
Normal pregnancy is associated with various physiologic and hemodynamic changes, especially during the last month very close to the delivery (1, 2), which might affect the response to drugs or anesthetics (3, 4). Decreased plasma cholinesterase (ChE) activity and increased oxidative stress (OS) biomarkers in the plasma malondialdehyde (MDA) have been reported in the last trimester of pregnancy (5-7). The decrease in plasma ChE activity might predispose pregnant women to the possibility of adverse drug reactions when neuromuscular blocking agents or anesthetics are used, especially in cesarean section (CS) delivery (8-10). A low level of plasma ChE is considered a risk factor in pregnant women (11), and the condition can be complicated when preeclampsia (pregnancy hypertension) coexists (12). On the other hand, OS biomarkers were reported to increase in pregnancy and were observed to be involved in the pathogenesis of preeclampsia complications (13, 14). The general anesthetic propofol and the spinal one bupivacaine are widely used in CS delivery (15, 16) and might affect plasma ChE activity and oxidative status (7, 17-19). These anesthetics even affect the quality of life after the CS delivery (20, 21).
A recent report implicates the possibility of the existence of health risks from reduced plasma ChE activity during propofol anesthesia and increased OS in women undergoing elective CS delivery (7). However, despite some in vivo findings on plasma ChE and MDA levels (5-7), limited information is available on the in vitro assessment of anesthetics on pregnant women’s plasma. The in vitro assessment of plasma ChE activity and OS biomarkers have been used to minimize invasive activities in patients or experimental animals (22-26). Furthermore, the in vitro experimental paradigms avoid the possibility of interference via maternal conditions involving anesthetics and surgical manipulations for CS delivery and the expected post-delivery biochemical changes (2, 6, 14).
2. Objectives
The purpose of the present study was to further explore and ascertain the effects of propofol and bupivacaine on plasma ChE activity and MDA level under in vitro conditions without in vivo complications.
3. Methods
3.1. Ethical Approval
Female subjects undergoing elective CS deliveries were recruited for the present study (age range: 20 - 45 years), as detailed in a previous study (7). The study subjects were informed about the purpose of the study, and written consent was obtained from each one. The Committee of Postgraduate Studies, College of Pharmacy, University of Duhok, KRG, Iraq, approved the present study (No. 470, October 6, 2021), and the Research Ethics Committee, Duhok Directorate General of Health, Duhok, KRG, Iraq, also confirmed its approval (No. 10112021-11-17, November 10, 2021). Furthermore, this study complied with the ethical standards of the Helsinki Declaration of Ethical Principles for Medical Research Involving Human Subjects, as revised in 2013.
3.2. Blood Sampling
Heparinized 5 mL venous blood samples were obtained only once from each of the 20 women undergoing elective CS just before the induction of anesthesia. The blood samples were centrifuged at 1037 g for 15 minutes to obtain plasma aliquots, which were stored frozen at –20ºC for the in vitro ChE and MDA assays.
3.3. Used Anesthetics
Propofol 1% (Polifarma, Istanbul, Turkey) and bupivacaine 0.5% (Aguettant Corporate, Lyon, France) were used for the experiments. In this study, none of the participants received anesthetics. Blood samples were taken from them once just before undergoing anesthesia for elective CS delivery. Then, all of the anesthetic in vitro experiments were conducted on pooled plasma samples of 20 pregnant women.
3.4. Measurements of Plasma ChE Activity and MDA Level
A modified electrometric method was used to measure plasma ChE activity (ΔpH/20 min at 37ºC) as described earlier using 0.2 mL plasma sample and 0.1 mL of the substrate acetylcholine iodide (7.1%) with an incubation time of 20 minutes (27, 28). The plasma MDA level was measured spectrophotometrically at 535 nm, as described earlier (29).
3.5. In vitro Inhibition of Plasma ChE Activity with Propofol or Bupivacaine
On the experimental day, 20 plasma samples were pooled for propofol or bupivacaine in plain glass containers and mixed before taking out plasma aliquots for the ChE assay. We used the method of 10-minute incubation of the inhibitor with the ChE source (plasma) at 37ºC (30, 31). The baseline and residual plasma ChE activity was measured electrometrically (27, 28). The inhibitor-ChE combination (n = 5/concentration) included propofol (0-baseline, 25 and 50 µM) (22, 32) and bupivacaine (0-baseline, 1.1 and 2.2 µM) (33) in the final reaction mixture (6.3 mL). The choice of these concentrations was based on preliminary experiments and the literature as mentioned above.
3.6. In vitro Antioxidant Property of Anesthetic Drugs by Measuring Plasma MDA Level
This method of using plasma samples for the evaluation of OS in in vitro tests was, in principle, according to the literature (23, 24). Pooled plasma samples were used with different concentrations of propofol or bupivacaine (n = 4/concentration). The OS in vitro test mixtures contained the plasma sample (0.25 mL), propofol at 0, 25, 50, and 100 µM, bupivacaine at 0, 1.1, 2.2, and 4.4 µM, or distilled water (-ve and +ve controls). The tubes containing the plasma and the anesthetic were incubated in a water bath at 37ºC for one hour. Thereafter, 0.1 mL of H2O2 (100 µM) as a source of OS was added (32, 34). The sample-mixture contents were then subjected to another one-hour incubation in a water bath at 37ºC. Thereafter, the level of MDA in the plasma was determined spectrophotometrically as described above.
3.7. Statistics
The data were statistically analyzed using the analysis of variance followed by Tukey’s test, using the software program PAST4.12 (https://www.nhm.uio.no/english/research/resources/past/). The level of accepted statistical significance was at P < 0.05.
4. Results
4.1. In vitro Plasma ChE Activity
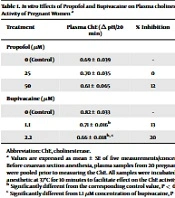
After incubating pooled plasma samples of pregnant women set for CS delivery with the anesthetics at 37ºC for 10 minutes, propofol at 25 and 50 µM did not significantly (P = 0.97 and 0.474, respectively) affect plasma ChE activity in vitro, when compared to the baseline-control value (Table 1). However, the in vitro incubation of the samples with bupivacaine at 1.1 and 2.2 µM, in a concentration-dependent manner, was significantly different (P = 0.02 and 0.001, respectively) and inhibited plasma ChE activity by 13% and 20%, respectively, in comparison to the corresponding control value (Table 1).
Abbreviation: ChE, cholinesterase.
a Values are expressed as mean ± SE of five measurements/concentrations. Before cesarean section anesthesia, plasma samples from 20 pregnant women were pooled prior to measuring the ChE. All samples were incubated with the anesthetic at 37ºC for 10 minutes to facilitate effect on the ChE activity.
b Significantly different from the corresponding control value, P < 0.05.
c Significantly different from 1.1 µM concentration of bupivacaine, P < 0.05.
4.2. In vitro Antioxidant Effects of Propofol and Bupivacaine on Plasma MDA Level
We used the in vitro method of incubation of plasma samples of pregnant women set for CS delivery by exposing samples containing different concentrations of anesthetics to 100 µM H2O2 for 1 hour at 37ºC. By using this in vitro experimental paradigm, the source of OS H2O2 elevated MDA level in the plasma, and prior treatments with both propofol and bupivacaine in a concentration-dependent manner reduced the MDA level when compared to respective control values (Table 2). Propofol at 25, 50, and 100 µM reduced MDA levels in the plasma samples by 6% (P = 0.933), 33% (P = 0.02), and 49% (P = 0.001), respectively (Table 2). Similarly, bupivacaine at 1.1, 2.2, and 4.4 µM reduced MDA levels in the plasma samples by 7% (P = 0.823), 17% (P = 0.202), and 45% (P < 0.001), respectively (Table 2).
Abbreviation: MDA, malondialdehyde
a Values are expressed as mean ± SE of four measurements/concentrations.
b Plasma samples were incubated in vitro with the anesthetics for 1 hour at 37ºC and then with 100 µM H2O2 for another hour at 37ºC.
c Significantly different from the corresponding control value, P < 0.05.
d Significantly different from 25 µM concentration of propofol, P < 0.05.
e Significantly different from 1.1 µM concentration of bupivacaine, P < 0.05.
f Significantly different from 2.2 µM concentration of bupivacaine, P < 0.05.
5. Discussion
The in vitro experiments of the present study supported the fact that plasma ChE activity could be inhibited by the use of bupivacaine but not by propofol. Local anesthetics, specifically bupivacaine, inhibit the butyryl ChE, which is a form of pseudo-ChE, also known as the plasma ChE (17, 35). A recent finding reported reduced plasma ChE activity in women who underwent elective CS with propofol and bupivacaine (7). These in vivo findings, however, do not differentiate the drug’s effect on blood ChE activity from those of the physiological changes observed in the last month of pregnancy (5, 36).
In light of the importance of plasma ChE, which is associated with the metabolism of anesthetic and neuromuscular blocking agents (36, 37), it seems reasonable to practice caution in dealing with CS cases due to the risk of reduced plasma ChE activity that occurs during pregnancy and from additional intrinsic input from the anesthetic itself, in this instant, bupivacaine. Furthermore, from the clinical standpoint, reduced plasma ChE in pregnancy (38) is a risk factor for women who might develop a condition of preeclampsia (11, 14), exerting additional burden on the body in dealing with neuromuscular blocking drugs (10, 37), anesthetics (8, 9), and even when exposed to pesticides (39).
As the increase in OS is an important biomarker of advanced pregnancy and its complications (12-14, 38), the unique finding of the present study was that both propofol and bupivacaine revealed an antioxidant effect by reducing MDA level after the incubation of the plasma sample with H2O2 as a source of oxidant. H2O2 has been used to induce OS both in vivo and in vitro using different cellular systems (32, 34). This study suitably used the plasma of the pregnant women just before the CS, a biological source for oxidation/antioxidation mechanisms, by monitoring the MDA level in vitro without the effects of the whole-body biochemical mechanisms on this OS biomarker (6).
The in vitro antioxidant effect of propofol correlates with its antioxidant effect reported after in vivo administration (7, 40-42). However, such an antioxidant effect has not been reported with bupivacaine, as reported in the present study; therefore, additional in vitro and in vivo studies are warranted with the use of different types of local anesthetics, especially those with anti-ChE activity.
The clinical relevance of the present study lies in the fact that it draws attention to the versatility of the in vitro experimental conditions in demonstrating reduced plasma ChE activity by the local anesthetic and showing the anesthetics’ antioxidant effects that could be important in avoiding possible drug-ChE interaction and for the follow-up of maternal antioxidant status in response to drugs/anesthetics, especially after the CS delivery. Further studies are also needed to determine the potential drug interactions of anti-ChE anesthetics in vitro using plasma samples from pregnant women.
5.1. Limitations
An important limitation of the present study is the lack of follow-up of the participants by conducting the same experimental protocol on blood samples after the CS delivery. Two common anesthetics, the general anesthetic propofol and the local anesthetic bupivacaine, were used in the present study. Further studies are needed on different types of anesthetics.
5.2. Conclusions
The in vitro experiments of the present study were versatile tools to assess the anti-ChE and anti-OS activity of the anesthetics, and the findings in the present study suggest that bupivacaine exerts anti-ChE activity that should be taken into consideration in CS anesthesia. Moreover, both propofol and bupivacaine possess antioxidant properties that need further assessment in clinical studies.