1. Introduction
Currently, artificial intelligence (AI) is becoming increasingly pervasive across various scientific fields, including medicine and healthcare. Medical applications of AI have seen significant growth in recent years (1, 2). Given the direct impact of medical decisions and activities on human life, the healthcare sector receives substantial attention from the research community. In this context, AI's potential to assist physicians and medical staff in managing complex tasks, handling a body of data, and making medical decisions has garnered considerable interest.
Numerous studies conducted in recent years have demonstrated the high capabilities of AI algorithms, resulting in a noticeable reduction of risks associated with medical practices. Furthermore, AI implementation has contributed to providing a better overall experience for both patients and medical staff alike.
The field of anesthesia is one of the critical areas in medical sciences. Since conscious patients are unable to breathe due to anesthetic drugs, the anesthesiologist must ensure they have stable breathing conditions through appropriate interventions. Therefore, making accurate decisions in this context is of utmost importance. Tasks such as predicting the depth of intraoperative anesthesia (3), developing metrics for neurological care (4), and predicting postoperative complications (5) are among the responsibilities that anesthesiologists perform with the assistance of AI algorithms. This integration of AI results in reduced errors, increased speed, and enhanced accuracy, as reported by anesthesiologists.
This study is a review of current trends in AI in anesthesia within 2020-2022. Moreover, this study provides substantial assistance for anesthesiologists who are familiar with the basic concepts of AI and machine learning (ML) and are interested in monitoring the latest developments in this field. Instead of focusing extensively on teaching the basics of ML and AI, the authors have dedicated their efforts to reviewing existing studies and covering a wider range of articles.
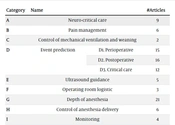
After reviewing the studies in the field of anesthesia and AI, existing studies were summarized by presenting a tabular structure of the main details within each study. This structure includes information on the purpose and type of surgery and anesthesia, the AI algorithms employed, the features used by AI models, dataset information, data accessibility, and evaluation criteria. Additionally, this study categorized and reviewed articles with a higher level of detail than Hashimoto et al.’s study (6), which divided the studies into six categories. This study extends the previous research by introducing three new categories and incorporating recent studies from 2020. The collected studies were thoughtfully divided into nine categories based on the expertise of the anesthesiologist (Table 1).
| Category | Names | #Articles | |
|---|---|---|---|
| A | Neuro-critical care | 9 | |
| B | Pain management | 6 | |
| C | Control of mechanical ventilation and weaning | 2 | |
| D | Event prediction | D1. Perioperative | 15 |
| D2. Postoperative | 16 | ||
| D3. Critical care | 12 | ||
| E | Ultrasound guidance | 5 | |
| F | Operating room logistic | 3 | |
| G | Depth of anesthesia | 21 | |
| H | Control of anesthesia delivery | 6 | |
| I | Monitoring | 4 | |
The remainder of the study is structured as follows: Section 2 provides an overview of AI and ML techniques. The categorization and reviews are presented in Section 3. A comprehensive discussion of the reviewed studies is presented in Section 4. Finally, Section 5 concludes the study and offers insights into potential future directions.
2. A Brief Introduction to AI and ML
Artificial intelligence is a branch of computer science whose main purpose is to produce intelligent machines capable of performing tasks that require human intelligence. This technology is a type of human intelligence simulation for computers, mainly aiming to design and build machines that can think like humans and imitate their behavior. Artificial intelligence techniques can be divided into several major categories, and currently, the two categories of ML and deep learning (DL) are widely used in various applications. This section provides a brief description of the concepts of ML and DL and the interpretability of learning models for the reader's general acquaintance.
Machine learning is considered one of the most important branches of AI. In ML, the learning process begins with observations in the form of data. The learner uses examples, direct experiences, or instructions to identify specific patterns and automatically make decisions and solve problems. Machine learning algorithms are typically categorized based on their learning styles, such as supervised learning, unsupervised learning (7), and semi-supervised learning, depending on the observability of variables under investigation.
Deep learning is a subset of ML that mimics the way the human mind learns about specific subjects. Deep learning aims to learn complex patterns by finding representations that fit each problem through successive layers of neural networks. Feature extraction is a key aspect of both ML and DL; however, DL algorithms are more automated than ML, where human resources might be involved in feature selection.
The interpretability of learning model outcomes is one of the most important issues in both ML and DL. When considering a particular medical problem, a learning algorithm can inform the physician of various predictions related to the problem. However, it might not provide the physician with sufficient information about the underlying reasons for those predictions and the process of reaching them. This “black box” nature of the learning algorithm might limit its applications in the medical field. To address this challenge, interpretability techniques are applied to the immediate results of the models. A model is considered interpretable when one can easily and significantly grasp the reasoning behind its predictions and decisions. More interpretable models are easier for human resources to understand and trust, especially in critical domains, such as healthcare (8).
3. Literature Review
This section studies all articles within a specific category based on their similarities.
3.1. Category A: Neuro-critical Care
The brain is the most vital organ of the human body. Therefore, specialists must pay special attention to brain function during anesthesia. Managing and controlling the function of the brain and other organs is done through neuro-critical care. Predicting and monitoring brain damage can be challenging for human resources, and as a result, AI algorithms have been used to create systems for performing such tasks. Brain injuries can be divided into two groups: Traumatic and non-traumatic. Traumatic injuries result from trauma or brain injury; nevertheless, non-traumatic brain injuries are caused by vascular accidents, such as rupture or bleeding in the brain or narrowing of the arteries (cerebral ischemia). Most studies in this category are related to traumatic brain and head injuries.
Intracranial hemorrhage is considered one of the most traumatic brain injuries. In two studies (9) and (10), deep neural networks and unsupervised ML algorithms were employed to analyze this injury, respectively. In another study by Schweingruber et al. (4), a deep, long, short-term memory (LSTM) neural network was used to predict the critical stages of intracranial hypotension and intracranial pressure, which are types of traumatic brain injuries. Farzaneh et al. (11, 12) conducted two studies focused on the use of AI methods to classify and predict different types of brain damage. In 2020, they used an ML model to assess the severity of subdural hematoma (11). In 2021, they provided long-term performance outcomes for patients with traumatic brain injury (TBI) by presenting an ML framework (12). The latter study’s results were interpreted using the Shapley method.
Seizures are bursts of uncontrolled electrical activity between brain cells, causing temporary abnormalities in the function of some organs. The random forest (RF) model was used to diagnose and monitor traumatic brain injuries related to seizures in a study (13); however, another study (14) used the generalized linear model (GLM). Both studies utilized continuous electroencephalogram (EEG) signals. The use of interpretability techniques is one of the advantages and positive contributions of these studies. Given the importance of interpretability in medical applications, as mentioned in section 3, it is noteworthy that its significance in the field of neurological care is amplified due to the presence of the most critical organ in the human body. Among the traumatic injuries of the head, subarachnoid hemorrhage (SAH) injuries were predicted by Koch et al. (15) using the Elastic-Net ML model and orthogonal partial least squares-discriminant analysis (OPLS-DA). Hypoxic-ischemic brain injury is a non-traumatic brain injury that can occur after cardiac arrest. In another study, Elmer et al. developed a new clustering algorithm called K-prototypes, inspired by the famous K-means clustering algorithm, to identify the phenotypes of primary brain damage after cardiac arrest (16). Further details about the reviewed studies in this category are shown in Table 2.
| No. | Study | Goal | Type of Brain Injury | Type of Anesthesia | Induction Drug(s) | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Xin et al. (2021) (9) | To evaluate the value of propofol anesthesia for brain protection of patients undergoing craniotomy evacuation of the hematoma | Cerebral hemorrhage | General | Sufentanil and cisatracurium | Request | 100/Yantai Yuhuangding Hospital | Extracted features from diffusion tensor imaging images that are a special form of MRI by residual block | DL super-resolution | Multiscale residual network (for experimental group) | FA values, NHISS scores, brain metabolism indexes at some time points, NSE and S100β protein levels, and the probability of postoperative complications in the corticospinal tract of the hind limb of the internal capsule of the affected side were better in the experimental group than in control group | No |
| 2 | Farzaneh et al. (2021) (12) | To predict long-term functional outcomes of TBI patients using available data | Traumatic brain injury (TBI) or “Silent Epidemic" | - | - | Request | 881 (M: 65, F: 224)/ProTECT III dataset | 18 EHR variables and medical history from which three sets of features are extracted, including all candidate variables, excluding non-robust variables, and excluding non-robust and counterintuitive variables | XGBoost | XGBoost on “All candidate variables" feature set | AUC: 0.809, accuracy: 75.3%, F1-score: 70.5%, sensitivity: 70.1%, specificity: 79.1%, precision: 70.9% | Yes, using Shapely |
| 3 | Koch et al. (2021) (15) | To ascertain potential insights into pathological mechanisms of injury after aSAH | Aneurysmal subarachnoid hemorrhage | - | - | Unavailable | 81 (M: 32, F:49) cerebro spinal fluid samples/not reported | Patient demographic and clinical characteristics, including World Federation of Neurological Surgeons grade, modified Fischer score, means of treatment, and need for permanent CSF diversion | Elastic Net ML and orthogonal partial least squares-discriminant analysis | EN and OPLS-DA | EN ML and OPLS-DA analysis identified 8 and 10 metabolites, respectively | No |
| 4 | Schweingruber et al. (2022) (4) | To predict critical phases of intracranial hypertension in patients with invasive ICP measurement | Evolution of ICP | - | - | External datasets are available at PhysioNet.org, and local datasets are available upon request. | 3978 including local dataset: ICP-ICU dataset (1346) and external datasets: (MIMIC-III (998) and eICU (1634))/not reported | Descriptives (age, weight, height, diagnosis) and most common and frequent features in all databases (vital signs, laboratory, medication, blood-gas analysis) | LSTM | LSTM | Using LSTM in this study had good results. | No |
| 5 | Bernabei et al. (2021) (13) | To present a real-time alerting and monitoring system for epilepsy and seizures that dramatically reduces the amount of manual electroencephalogram review | Epilepsy and seizures | - | Thiopental, midazolam, ketamine | Available | 97 (M: 44, F:53)/ICUs at the University of Pennsylvania Health System | Continuous EEG signals: Power in the delta, theta, alpha, beta frequency bands, signal line length, wavelet entropy, statistical features of the signal, the mean value of the upper signal envelope of the electroencephalogram waveform | RF | RF | Mean seizure sensitivity: 84% (cross-validation) and 85% (testing), mean specificity: 83% (cross-validation) and 86% (testing) | Yes, using RF. |
| 6 | Narula et al. (2021) (10) | To detect bursts in EEG and generate burst-per-minute estimates for the purpose of monitoring the sedation level in an ICU | Intracranial hemorrhage | - | Isoflurane | Unavailable | 29 (M: 16, F:13)/Neurocritical Care Unit, University Hospital Zurich | Continuous EEG signals: Distance between covariance matrices | BSUPP (new unsupervised burst suppression detection algorithm) | BSUPP | Mean absolute error in bursts per minute: 0.93, average of Sensitivity: 81%, average of specificity: 81%, AUROC: 0.82, average NPV: 97% | No |
| 7 | Fumeaux et al. (2020) (14) | To create a seizure-detection approach | Spontaneous seizures | - | - | Unavailable | 112/focal epilepsy dataset and multifocal epilepsy dataset | (Continuous EEG) cEEG signals: RMS of signal, coastline, skewness, kurtosis, autocorrelation function, Hjorth parameters (activity, mobility, complexity of EEG signal), maximal cross-correlation, and extra | GLM | GLM | AUROC: 0.890 latency to detection: Under 5 seconds for over 80% of seizures and under 12 seconds for over 99% of seizures | Yes, using the logit link function |
| 8 | Farzaneh et al. (2020) (11) | To segment and assess the severity of subdural hematoma for patients with TBI | TBI | Sedation | Sedation with Propofol or dexmedetomidine, analgesia with fentanyl | Unavailable | 11/Michigan Medicine Neurological Intensive Care Unit or Emergency Department | Computed tomography scans: Age, location-based (radial distance, Azimuth angle, elevation angle, distance to skull), histogram-based (minimum, maximum, average, SD, skewness, kurtosis, entropy), filtering-based (Gabor, Laplacian of Gaussian), deep features | RF | RF+Post-processing | Recall: 98.81%, specificity: 92.31%, F1-score: 98.22% | No |
| 9 | Elmer et al. (2020) (16) | To detect early post-cardiac-arrest brain injury phenotypes | Hypoxic-ischemic brain injury | Sedation | Sedation with propofol or dexmedetomidine, analgesia with fentanyl | Available | 1086 (M: 613, F:437)/not reported | Neurological examination, EEG, and brain CT imaging | K-prototypes | K-prototypes | Survival to hospital discharge: 27% | Yes, using the center of clusters |
Abbreviations: MRI, magnetic resonance imaging; DL, deep learning; FA, fractional anisotropic; NHISS, National Institute of Health Stroke Scale; NSE, neuron-specific enolase; TBI, traumatic brain injury; EHR, electronic health records; XGBoost, extreme gradient boosting; AUC, area under the curve; aSAH, aneurysmal subarachnoid hemorrhage; CSF, cerebrospinal fluid; ML, machine learning; EN, elastic Net; OPLS-DA, orthogonal partial least squares-discriminant analysis; ICP, intracranial pressure; ICU, intensive care unit; LSTM, long short-term memory; EEG, electroencephalogram; RF, random forest; BSUPP, unsupervised burst suppression detection algorithm; AUROC, area under the receiver operating characteristics; NPV, negative predictive value; GLM, generalized linear model; CT, computed tomography.
3.2. Category B: Pain Management
Pain is a sensation caused by stimulating nociceptors in the central or peripheral nervous system. This feeling can arise following a surgical incision and might result from inadequate anesthetic drug injection during an operation or insufficient postoperative analgesia. Therefore, pain management and prevention are of great importance for both the anesthesiologist and the patient. Artificial intelligence models can assist specialists in better pain management by measures, such as defining the pain index and predicting its timing. The reviewed studies in this category can be divided into two subcategories.
The first subcategory includes studies aiming to diagnose and predict the occurrence of pain during or after an operation. For example, in a study (13), the possibility of diagnosing toothache based on the three signals of electrocardiography (ECG), photoplethysmography (PPG), and chest were investigated using the RF model, which performed well on the test dataset. Tan et al. (17) compared ML techniques to statistical inference techniques to identify and predict breakthrough pain during labor, with the ML models not performing better than the statistical methods, potentially due to the presence of unbalanced data.
The second subcategory of studies focuses on assessing the level of pain by defining a pain index. The lack of a well-defined criterion for determining and measuring a patient's pain level to adapt drug injections during general anesthesia is a major challenge. Gonzalez-Cava et al. (18) aimed to evaluate the performance of the pain index using ML classifiers; however, another study (19) indicated that monitoring the injectable drug dose using the pain level index helped reduce postoperative pain. The Nociception level (NOL) index is another multi-parameter AI-based index designed to monitor pain during general anesthesia, which was observed to reduce postoperative pain. In another study (20), a new relief index was developed using photoplethysmogram spectroscopy and a convolutional neural network (CNN) to assess pain in conscious patients.
Rebound pain is a common outcome that occurs after a peripheral nerve block, usually subsiding 24 to 48 hours after the block was formed, often occurring after outpatient operations for patients. To address this issue, Barry et al. (21) used ML models to examine factors associated with rebound pain in patients who received peripheral nerve blocks for outpatient operations. A metric called the numerical rating scale (NRS) was defined to measure the level of pain in this study. The logistic model tree attribute-selected classifier with receiver operating characteristic (ROC) showed the best-reported result at around 60%. Table 3 shows the main points of the reviewed studies in this category.
| No. | Study | Goal | Type of Surgery | Type of Anesthesia | Induction Drug(s) | Evaluation Pain Index | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tan et al. (2021) (17) | Identifying parturients at increased risk of breakthrough pain during labor epidural analgesia | Parturition | Regional | Fentanyl and ropivacaine. | - | Unavailable | 20798/KK Women’s and Children’s Hospital, a tertiary obstetric hospital | Maternal age, race/ethnicity, BMI, ASA PS score, parity, twins, pre-neuraxial analgesia pain score, pre-neuraxial analgesia cervical dilation, post-neuraxial analgesia highest pain score (0-10), analgesia use prior to neuraxial analgesia, neuraxial technique, combined spinal-epidural, number of neuraxial attempts, median, neuraxial procedure total time, median, depth to epidural space, median, length of catheter in epidural space, median and etc. | RF, XGBoost & LR | LR | Sensitivity: 69.4%, specificity: 73.3%, PPV: 30.1%, NPV: 93.5% |
| 2 | Barry et al. (2021) (21) | To investigate the incidence and factors associated with rebound pain in patients who received a PNB for ambulatory surgery | Ambulatory surgeries | Local (peripheral nerve block) | Ropivacaine (or bupivacaine) with lidocaine. | Numerical rating scale (NRS) | Unavailable | 972/Hospital databases Draagerwerk AG & Co | Age, BMI, gender, surgery duration, local anesthetic volume, local anesthetic dose, sensory block duration, motor block duration, ASA physical status, surgical site, surgical site (specific), surgery type, general anesthesia, peripheral nerve block type, local anesthetic drugs, analgesia adjuncts, postoperative NSAID use, postoperative acetaminophen use, postoperative opioid use | Univariate linear regression, multivariable LR, logistic model tree attribute-selected classifier | Logistic model tree attribute-selected classifier | ROC: 0.6 |
| 3 | Choi et al. (2021) (20) | Develop a new analgesic index to objectively assess pain in conscious patients. | Breast, colorectal, hepatobiliary, stomach, thyroid | General | Propofol and remifentanil | Spectrogram–CNN index | Unavailable | 100 (M:44, F: 56)/not reported | Photoplethysmogram spectrograms, gender, age, height, weight, ASA | CNN | CNN | AUC: 0.76 balanced accuracy: 71.4%, sensitivity: 68.3%, specificity: 73.8% |
| 4 | Gonzalez-Cava et al. (2020) (18) | Evaluate the suitability of the analgesia Nociception index as a guidance variable to replicate the decisions made by the experts when a modification of the opioid infusion rate is required. | Cholecystectomy surgery | General | Remifentani and propofol | Analgesia Nociception index (ANI) | Unavailable | 17 (M: 4, F:13)/Hospital Universitario de Canarias | Feature vector proposal 1: Hemodynamic information (SP, SP5, SP10 DP, DP5 DP10 HR, HR5, HR10 Remi, Remi5, Remi10) Feature vector proposal 2: Minimum ANI information (SP, SP5, SP10, DP, DP5 DP10, HR, HR5, HR10, Remi, Remi5, Remi10, and extra, | KNN, DT, LDA, SVM, LR, ensemble classifiers | SVM | Accuracy: 86.21%, precision: 86.11%, recall: 91.18%, specificity: 79.17%, AUC: 0.89 Kappa index: 0.71 |
| 5 | Teichmann et al. (2020) (22) | Detection of dental pain sensation based on cardiorespiratory signals using a machine learning classifier | Dental treatment | General | - | - | Unavailable | 20 (M: 16, F:4)/Department of Prosthodontics and Biomaterials-Center of Implantology, Medical Faculty, RWTH Aachen University | Frequency spectral bins, levels of the discrete wavelet transform, average height, maximum deviation in height, average pulse beat-to-beat time, maximum deviation in beat-to-beat times, average area, the maximum deviation of areas, the average ratio between pulse width and height, the maximum deviation of the ratio between pulse width and height | RF | RF | Sensitivity: 87%, specificity: 63%, AUC: 0.828 |
| 6 | Meijer et al. (2020) (19) | To reduce postoperative pain using Nociception level-guided opioid dosing during general anesthesia | Abdominal surgery | General | Fentanyl & sevoflurane | Nociception level (NOL) index | Unavailable | 50 (M: 22, F:28)/Leiden University Medical Centre, Alrijne Hospital | Age, gender, weight, height, BMI, MAP, HR, ASA physical status, general surgery, gynecology, urology | NOL-guided dosing, standard care dosing | NOL-guided Dosing | Median postoperative pain score: 3.2 postoperative morphine consumption (SD): 0.06 (0.07) |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiology; CSF, cerebrospinal fluid; CSE, combined spinal-epidural analgesia; RF, random forest; XGBoost, extreme gradient boosting; LR, logistic regression; PPV, positive predictive value; NPV, negative predictive value; HER-2, human epidermal growth factor receptor-2; DT, decision tree; GB, gradient boosting; LightGBM, light gradient boosting machine; AUC, area under the curve; PNB, peripheral nerve block; ROC, receiver operating characteristics; CNN, convolutional neural network; ASA PS, American Society of Anesthesiologists Physical Status; PACU, post anesthesia care unit; ANI, analgesia nociception index; KNN, K-nearest neighbor; LDA, linear discriminant analysis; SVM, support vector machine; NOL, nociception level; MAP, mean arterial pressure; HR, heart rate.
3.3. Category C: Control of Mechanical Ventilation and Weaning
Mechanical ventilation is a life-supporting treatment that aids patients who are unable to breathe on their own. It involves the use of a mechanical device, such as a ventilator, artificial respiration device, or respiratory system, to assist patients in breathing. Patients requiring respiratory support due to a serious illness are typically hospitalized in the intensive care unit (ICU). However, mechanical ventilation can pose challenges, such as patient restlessness caused by the use of lighter anesthesia and inadequate oxygen supply to the respiratory organs. Artificial intelligence models have been employed to address these challenges effectively.
Two reviewed studies in this category utilized ML algorithms to predict and manage challenges related to patient restlessness due to lighter anesthesia and insufficient oxygen supply to the respiratory organs. The use of lighter sedatives with lighter anesthetics is often recommended to improve aggressive mechanical ventilation, reduce mortality, and enhance clinical outcomes. However, this approach can lead to issues, such as accidental extubation and patient-ventilator asynchrony. Additionally, the use of lighter sedatives might increase the risk of patient agitation in response to other nervous stimulation. To tackle these challenges, timely prediction of patient agitations and their management is crucial when using lighter anesthesia. Therefore, one study (23) developed a collective ML model to predict patient agitation in the ICU over the next 24 hours.
Another significant aspect of mechanical ventilation is assessing spontaneous breathing (SB) attempts, which is an essential criterion in respiratory drive. However, SB levels can vary due to various factors, including evolving pathology and sedation levels. Therefore, the continuous assessment of SB is necessary. In a study (24), a convolutional autoencoder (CAE) was developed to quantify the amount of SB using airway pressure and flow waveform data. The characteristics of each reviewed study in this category are summarized in Table 4.
| No. | Study | Goal | Type of Anesthesia | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhang et al. (2021) (23) | Prediction of agitation in invasive mechanical ventilation patients under light sedation. | Sedation | Unavailable | 578/Some ICUs in 80 Chinese hospitals | Risk factors for delirium identified, ventilator parameters that can influence asynchrony, including ventilation mode, positive end-expiratory pressure, plateau pressure, Fio2, respiratory rate, and minute ventilation | Adaboost, Linear SVM with Class Weights, C5.0, XGboost, An ensemble model including four mentioned models | Ensemble model | AUC: 0.918 | Yes, using the “BreakDown” algorithm |
| 2 | Ang et al. (2021) (24) | To quantify the magnitude of spontaneous breathing (SB) effort using only bedside (mechanical ventilation) MV airway pressure and flow waveform | - | Unavailable | 13.6M+1800/simulated SB flow and normal flow data (NB)+National University of Singapore Hospital (test data) | SB flow | Convolutional autoencoder | Convolutional auto encoder | MSE: 4.77 | No |
Abbreviations: ICU, intensive care unit; SVM, support vector machine; XGboost, extreme gradient boosting; AUC, area under the curve; SB, spontaneous breathing; NB, normal breathing; MSE, mean square error.
3.4. Category D: Event Prediction
This category examines studies that aim to predict events, which involve estimating the probability of specific occurrences in the future. Artificial intelligence algorithms have been employed as tools to enhance the accuracy, ease, and speed of predicting these events and preventing related complications. The events are categorized into three subcategories: Perioperative, postoperative, and critical care, each of which will be discussed in more detail below.
3.4.1. Subcategory D1: Perioperative
Perioperative events refer to occurrences that might happen to a patient before, during, or immediately after an operation. In this subcategory, the prediction of such events is the focus (25, 26). A common perioperative event is fluctuations in blood pressure, particularly hypotension, which can lead to serious complications, such as cardiovascular injury or even death. Several articles in this subcategory predicted hypotension before its occurrence to enable specialists to take necessary tasks to prevent it (27-33). Another crucial event is difficult laryngoscopy, defined as the inability to visualize part of the vocal cords during multiple laryngoscopy attempts by a trained anesthesiologist. Predictive models for difficult laryngoscopy were developed ML techniques in the studies of this subcategory (34, 35).
Additionally, Mathis et al. (36) utilized ML approaches to identify patients who ultimately faced postoperative heart failure with reduced ejection fraction (HFrEF). The aforementioned study demonstrated that the extreme gradient boosting algorithm outperformed other ML algorithms in this prediction task. Other studies in this subcategory applied DL to improve the detection of life-threatening arrhythmia (37), classify ECG signals for anesthesia assessment (38), and investigate the elements of synaptic transmission based on the anesthetized patient’s EEG data (39).
3.4.2. Subcategory D2: Postoperative
The postoperative period encompasses events occurring at long intervals after an operation (40, 41). In most of the reviewed articles in this subcategory, predicted events include postoperative complications in specific conditions or diseases.
Postoperative delirium was predicted in three studies using ML algorithms (5, 42). In addition to predicting delirium, several studies in this subcategory utilized ML algorithms to predict blood pressure fluctuations during the postoperative period. Palla et al. (43) and Schenk et al. (44) predicted postoperative hypotension; however, another study predicted an increase in postoperative hypertension (45). Other studies used ML techniques to predict postoperative complications, such as cardiac events (46), cerebral infarction and myocardial infarction (47), and acute kidney injury (48). Cao et al. (49) employed DL algorithms to predict serious complications after bariatric surgery. Qian et al. (50) presented a study evaluating the importance of operation time in classifying surgical complications using interpretable ML approaches.
Moreover, one study (41) introduced a tool called the surgical and medical postoperative complications prediction tool (SUMPOT) based on an artificial neural network to identify patients at risk of postoperative complications. Additionally, the relationship between cannabis use and a slight increase in the risk of postoperative nausea and vomiting was investigated using ML (51). Two studies in 2021 by Lu et al. (52, 53) focused on identifying patients in need of anterior cruciate ligament reconstruction (ACLR) (52) and predicting the cost of ACLR (53).
3.4.3. Subcategory D3: Critical Care
Studies in this subcategory primarily focused on predicting events related to clinical interventions for patients frequently admitted to the ICU. The worst postoperative event in this subcategory is patient death (54, 55). Several studies, similar to the previous subcategories, predicted hypotension by considering clinical interventions. Cherifa et al. (56) predicted hypotension in the ICU using deep neural networks; nevertheless, two other studies employed different ML algorithms for the same prediction (57, 58). Additionally, Hu et al. (59) used ML techniques to develop a model for predicting seizures in critically ill children. Myasthenia gravis (MG), a neuromuscular disorder associated with acquired autoimmunity causing muscle weakness, was also investigated in this subcategory, where Chang et al. (60) developed a decision tree-based model to predict the severity of MG.
Furthermore, predicting tracheal intubations was considered crucial in the ICU, especially for medical personnel not familiar with the procedure. Hayasaka et al. (61) designed an AI model using a CNN to classify difficult intubations based on the patient's facial image. Machine learning methods and statistical techniques were also used to investigate the relationship between positive cultures during hospitalization and long-term outcomes in critically ill surgical patients (62), the relationship between red cell distribution width (RDW) and prognosis in patients with sepsis-associated thrombocytopenia (SAT) (63), and the relationship between primary brain magnetic resonance imaging (MRI) data and functional outcomes of patients with severe herpes simplex encephalitis (HSE) 90 days after ICU admission (64). Moreover, a study (65) explored parametric and non-parametric methods for predicting cerebral performance category (CPC) using longitudinal data after cardiac arrest. Further detailed information about the reviewed studies in this category can be found in Tables 5, 6, and 7.
| No. | Subcategory | Study | Goal | Type of Surgery | Type of Anesthesia | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D1 | Maheshwari et al. (2020) (27) | To evaluate the performance of the hypotension (MAP < 65 mmHg for at least 1 min) prediction index algorithm derived from non-invasive arterial pressure waveforms in moderate-to-high-risk non-cardiac surgical patients | Non-cardiac | General | Unavailable | 305/ClearSight, Edwards Lifesciences | Waveform features and the patient demographics, including age, gender, height, and weight. | HPI algorithm for the 5, 10, and 15 minutes of prediction time points before each hypotensive episode for blinded arm, unblinded arm, combined groups | HPI algorithm for 5 min prediction time point before hypotension episode for blinded arm | AUC: 0.94, sensitivity: 86%, specificity: 87% | No |
| 2 | D1 | Li et al. (2021) (28) | Prediction of post-induction hypotension (SBP < 90 mmHg or MBP < 65 mmHg) in patients undergoing cardiac surgery | Cardiac | General | Unavailable | 3030/The Second Affiliated Hospital of Hainan Medical University | Preoperative variables including age, gender, BMI, underlying disease, EuroSCORE I, and ASA score; experimental findings including hemoglobin, serum creatinine, and total bilirubin; data on the patient's preoperative medications, such as the use of beta-blockers, insulin, aspirin, intraoperative medications and data on perioperative blood pressure | RF | RF | AUC: 0.843 | Yes, using the interpretability of RF |
| 3 | D1 | Frassanito et al. (2020) (29) | To assess the diagnostic ability of Hypotension Prediction Index (HPI) working with non-invasive ClearSight system in predicting impending hypotension (MAP < 65 mmHg for > 1 min) in patients undergoing major gynecologic oncologic surgery | Gynecologic oncologic | General | Unavailable | 28/Edwards Lifesciences HemoSphere platform | Extracted features from non-invasive arterial pressure waveform of ClearSight | HPI algorithm for the 5, 10, and 15 minutes of prediction time points before each hypotensive episode. | HPI algorithm for 15 min prediction time point before hypotension episode | AUC [95% CI]: 0.95, sensitivity [95% CI]: 85%, specificity [95% CI]: 85%, positive predictive value [95% CI]: 75%, negative predictive value [95% CI]: 91% | No |
| 4 | D1 | Gratz et al. (2020) (30) | To predict the likelihood of a given patient developing significant hypotension (SBP < 90 mmHg) under spinal anesthesia when undergoing a cesarean section (C/S) | Cesarean | Local | Unavailable | 45/not reported | Extracted features from signals using neural network model physiological data, including systole, diastole, mean arterial pressure (MAP), heart rate, and the AS parameter, on a beat-by-beat basis. | NN | NN | AUC: 0.87 | No |
| 5 | D1 | Lee et al. (2020) (31) | To predict hypotension (SBP < 90 mmHg or MBP < 65 mmHg) after tracheal intubation after intubation one minute in advance | Underwent laparoscopic cholecystectomy | General | Unavailable | 282/Soonchunhyang University Bucheon Hospital | Totally we had two kinds of features in this study: Raw features and statistical features, including electronic health records (demographic data, comorbidities, baseline) and vital recorder (mechanical ventilation data, bispectral index, anesthetic drug, vasoactive drug administration, Some information about hypotension) | Meta-learning models, such as RF, XGboost, DL models, especially CNN and DNN | Raw features: CNN Statistical features: RF | Accuracy of CNN for raw features: 72.6%, accuracy of RF for statistical features: 74.8% | Yes, using the feature importance of RF |
| 6 | D1 | Kang et al. (2021) (32) | To predict hypotension (SBP < 90 mmHg or MBP < 65 mmHg) in late Post Induction Hypotension (PIH) by using data in the early PIH part | Laparoscopic cholecystectomy | General | Available | 222/Soonchunhyang University Bucheon Hospital | In this study, 4 feature sets were created by different methods of feature selection, including feature set A (Min heart rate, Max volume of propofol), feature set B (mean volume of remifentanil, respiratory rate mean), feature set C (hypotension frequency, Max plasma concentration of propofol), all features (Min effect-site concentration of propofol, Max target concentration of propofol) | Four ML models, including NB, LR, RF, ANN | RF | Accuracy (Feature set C): 79.4%, precision (Feature set C): 81.1%, recall (Feature set B): 84.5%, AUC (Feature set C): 0.842, 95% CI (Feature set C): 0.736-0.948 | Yes, using the feature importance of RF |
| 7 | D1 | Wijnberge et al. (2020) (33) | To predict hypotension (MAP < 65 mmHg for at least 1 min) shortly before it occurs has been developed and validated | Elective noncardiac | General | Unavailable | 60 (M: 36, F: 24)/Amsterdam University Medical Centers, Location | Extracted features from the signal (patients based on characteristics divided into intervention and control groups) | HPI algorithm for the 5, 10, and 15 minutes of prediction time points before each hypotensive episode. | HPI algorithm for 15 min prediction time point before hypotension episode | The median time-weighted average of hypotension: 0.10 mm Hg (intervention group); 0.44 mm Hg (control group) | No |
| 8 | D1 | Solomon et al. (2021) (25) | To predict the occurrence of clinically significant intraoperative bradycardia at time points during an operative course by utilizing available preoperative electronic medical records and intraoperative anesthesia information management system data | Non-cardiac | General | Unavailable | 62182/ University of Washington Medical Center | Extracted features from time series signal | Build three models named TP1, TP2 & TP3 by using: GBM & LR | GBM | AUC: 0.89, specificity: 95%, sensitivity: 53%, PPV: 15%, NPV: 99% | Yes, using predictor variables of GBM |
| 9 | D1 | Jalali et al. (2021) (26) | To predict blood product transfusion requirements for individual pediatric patients undergoing craniofacial surgery | Craniofacial surgery | - | Request | 2143/Pediatric Craniofacial Surgery Perioperative Registry | Demographic and preoperative features | Six ML classification and regression models, including RF, AdaBoost, NN, GBM, SVM, Elastic Net methods | GBM | In classification: Sensitivity: 92% ± 3%, specificity: 89% ± 4%, F1-score: 91% ± 4%, AUROC: 0.87 ± 0.03, in regression: MSE: 1.15 ± 0.12, R-squared: 0.73 ±0.02, RMSE: 1.05 ± 0.06 | Yes, using the feature ranking of GBM |
| 10 | D1 | Kim et al. (2021) (34) | To develop and validate practical predictive models for difficult laryngoscopy | - | - | Unavailable | 616/Hallym University Chuncheon Sacred Heart Hospital | Age, Mallampati grade, BMI, Sternomental distance, neck circumference | MLP, LR, SVM, RF, XGBoost, LightGBM | LGBM | AUROC: 0.71 Sensitivity: 85% | No |
| 11 | D1 | Kim et al. (2021) (35) | To predict difficult laryngoscopy of neck circumference and thyromental height | - | General | Request | 1677 (M: 925, F: 752)/Hallym University Chuncheon Sacred Heart Hospital | Age, gender, height, weight, BMI, neck circumference, thyromental height | MLP, LR, SVM, RF, XGBoost, LightGBM | RF | AUROC: 0.79 AUPRC: 0.32 | No |
| 12 | D1 | Bollepalli et al. (2021) (37) | To improve life-threatening arrhythmia detection in the ICUs | - | - | Request+https://physionet.org/content/challenge-2015/1.0.0/ | 410/ICUs of Massachusetts General Hospital and PhysioNet | Deep features+ECG, blood pressure, PPG features (periodicity measure, sharpness measure, correlation measure, peak height stability measure, and extra. | Hybrid CNN | Hybrid CNN | Accuracy: 87.5% ± 0.5%, score: 81% ± 0.9%, evaluation on PhysioNet 2015 Challenge database: Accuracy: 84.3%, score: 93.9% | No |
| 13 | D1 | Yeh et al. (2021) (38) | To classify ECG image types to assist in anesthesia assessment | - | - | Available | 54190/MIT-BIH Arrhythmia Database | 2D ECG images | ResNet, AlexNet, SqueezeNet | ResNet | Accuracy: 97%, recall: 97%, precision: 97, F1-score: 97%, Kappa statistics: 0.96 | No |
| 14 | D1 | Hadjipavlou et al. (2021) (39) | Exploring elements of synaptic transmission, looking for possible contributions to the anesthetized EEG | - | General | Unavailable | Not reported/ Oxford University Clinical Academic Graduate School | Simulated electrocorticography: Alpha band at rest, loss of frequencies at induction, alpha and slow wave bands at maintenance, and broad spectral activity at emergence. AG, anesthetic GABA | Hodgkin-Huxley-type NN computer simulation | Hodgkin-Huxley-type NN computer simulation | - | No |
| 15 | D1 | Mathis et al. (2020) (36) | Identifying patients ultimately diagnosed with heart failure with reduced ejection fraction following surgery using preoperative and intraoperative data | Noncardiac surgery | General | Unavailable | 67697 (M: 32200, F: 35497)/ Multicenter Perioperative Outcomes Group (MPOG) database+Epic Systems | 628 preoperative and 1195 intraoperative features | L1 Regularized LR, RF, XGBoost | XGBoost | AUROC: 0.873, AUPRC: 0.040, accuracy: 80.82%, sensitivity: 80.84%, specificity: 80.82%, PPV: 1.78%, NPV: 99.90% | Yes, using the feature importance |
Abbreviations: MAP, mean arterial pressure; HPI, hypotension prediction index; AUC, area under the curve; SBP, systolic blood pressure; MBP, mean blood pressure; BMI, body mass index; ASA, American Society of Anesthesiology; RF, random forest; AS, arterial stiffness; NN, neural network; XGboost, extreme gradient boosting; DL, deep learning; CNN, convolutional neural network; DNN, deep neural network; PIH, post induction hypotension; ML, machine learning; NB, naïve bayes; LR, logistic regression; GBM, gradient boosting machine; PPV, positive predictive value; NPV, negative predictive value; SVM, support vector machine; AUROC, area under the receiver operating characteristics; MSE, mean square error; RMSE, root mean square error; MLP, multi-layer perceptron; LGBM, light GBM; AUPRC, area under the precision-recall curve; ICU, intensive care unit; ECG, electrocardiogram; PPG, photoplethysmography; HR, heart rate; EEG, electroencephalogram; GABA, gamma-aminobutyric acid.
| No. | SubCat. | Study | Goal | Type of Surgery | Type of Anesthesia | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D2 | Racine et al. (2021) (42) | To predict delirium in a rigorous and well-characterized, prospective, observational cohort study of delirium | Elective non-cardiac including | - | Unavailable | 560/Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, and Hebrew SeniorLife | Medical records includig: Surgical procedure, anesthesia type and duration, baseline diagnoses and comorbidity, abnormal laboratory results, development of delirium, precipitating factors for delirium (e.g., medications, iatrogenic events, catheters, or physical restraints), postoperative complications, and intercurrent illnesses | GB, Cross-validated LR, NN, RF, and Regularized Regression (least absolute shrinkage and selection and ridge regularization) & two ensemble approaches | Cross-validated LR for full feature set | AUC: 0.7; Sensitivity: 46%; Specificity: 81%; PPV: 43%; NPV: 83% | No |
| 2 | D2 | Lu et al. (2021) (52) | To identify patients requiring admission following elective anterior cruciate ligament reconstruction | Non-elective | Different type of anesthesia were used, including: Epidural, General, MAC/IV sedation, Regional, Spinal, Operative time | Unavailable | 4709/The ACS National Surgical Quality Improvement Program database | age, Gender, BMI, functional status, level of dyspnea, ASA Physical Status Classifcation, location from which patient was admitted, anesthesia type, operative time, admission quarter, diabetes mellitus, congestive heart failure, chronic obstructive pulmonary disease, smoking history, preoperative sepsis, preoperative use of a ventilator, ascites, wound infection, weight loss>10%, etc. | RF, XGBoost, LDA, AdaBoost & An additional model was produced as a weighted ensemble of the four fnal algorithms | Ensemble model | AUC: 0.76 | Yes |
| 3 | D2 | Lee et al. (2021) (40) | To learn patterns related to risk of in-hospital mortality for patients undergoing surgery under general anesthesia | - | General | Unavailable | 59985/UCLA Medical Center’s Perioperative Data Warehouse | Medical information including: Age, Estimated blood loss, Presence of arterial line, Presence of pulmonary artery line, Presence of central line, ASA score and other & Healthcare Cost and Utilization Project (HCUP) Code Descriptions including: UPPER GASTROINTESTINAL ENDOSCOPY, BIOPSY 3864, COLONOSCOPY AND BIOPSY, LAMINECTOMY, EXCISION INTERVERTEBRAL DISC and other | Generalized Additive Models with NN (GAM-NN) & LR | GAM-NN | AUC: 0.921; AP: 17.6% | Yes, using Interpretable model (GAM-NN) |
| 4 | D2 | Schenk et al. (2021) (44) | To investigate the effect of Hypotension Prediction Index-guided intraoperative haemodynamic care on depth and duration of postoperative hypotension | Elective noncardiac | General | Unavailable | 54/Amsterdam University Medical Centers | Extracted features from the invasive Blood Pressure signals | HPI algorithm | HPI algorithm | Intraoperative HPI-guided haemodynamic care did not reduce the TWA of postoperative hypotension | No |
| 5 | D2 | Tan et al. (2021) (45) | Prediction of early phase postoperative hypertension requiring the administration of intravenous vasodilators after carotid endarterectomy | - | General | Unavailable | 367/Huashan Hospital of Fudan University | Patient demographics, CEA procedure details, parameters of laboratory examination, imaging study & perioperative blood pressure | GBR Trees | GBR Trees | Average AUC: 0.77; Average Specificity: 52%; Sensitivity ~ 90% | Yes, using feature importance of GBRT |
| 6 | D2 | Lu et al. (2021) (53) | To predict cost after anterior cruciate ligament reconstruction | Ambulatory ACLR | Different types of anesthesia were used, including: MAC/IV sedation, Local anesthesia, General anesthesia & Regional anesthesia | Unavailable | 7311/New York State Ambulatory Surgery and Services database | Features included in initial models consisted of patient characteristics (age, Gender, insurance status, income, medical comorbidities as classified by the Clinical Classifications Software diagnosis code) as well as intraoperative variables (type of anesthesia and procedure-specific factors) | Four ML models including: RF, XGBoost, Elastic Net Penalized Regression & SVMs with radial kernels | RF | Accuracy: 87.8%; AUC: 0.848; Calibration and the Brier score: 20.8% | Yes, using interpretability of RF |
| 7 | D2 | Palla et al. (2022) (43) | To predict hypotension in the recovery area better than clinicians using readily available clinical information | Different type of surgery like Orthopaedic, General, Urology, ENT, etc | - | Unavailable | 121904/Two UW hospitals | Demographics data, Procedure details, Comorbidities, Vitals, Drugs & other | GBRT | GBRT | AUROC: 0.82; AUPRC: 0.4 | Yes, using ShAP Value |
| 8 | D2 | Jeong et al. (2021) (46) | To predict postoperative complications, major adverse cardiac events, for patients who underwent any type of surgery | Any type of surgery | General | Request | 586/Soonchunhyang university Seoul hospital | pre-op EMR features: demographic values (e.g., height, weight, Gender, age, BMI), several pre-op evaluation results (e.g., EF, PFT), pre/post hemodialysis evaluations (e.g., Na, K, Cl), and comorbidities (e.g., hypertension, atrial fibrillation) peri-op features: Anesthesia-related values (e.g., ASA, EM emergency operation, anesthesia method), and other operation-related values (e.g., anesthesia time, operation time, infusion of crystalloid or colloid) text features: Generated by applying NLP techniques to preanesthetic assessment documents | SVM, DT, RF, Gaussian NB, ANN, LR, XGBoost | RF | F1-score: 79.7% | Yes, using Recursive Feature Elimination (RFE) and K-best |
| 9 | D2 | Qian et al. (2021) (50) | To assess the significance of operative timing on classifying surgical complications | Different type of surgery like Obstetric, Gynecological, Liver, etc. | All types of anesthesia | Request | 107481(M:55515,F:51966)/University-affiliated, tertiary teaching hospital | Date and Time the Surgery, Duration of Surgery, Length of Stay, Surgical Discipline, Patient Age and Gender, Admission and Discharge Consultation Summaries, Preoperative Comorbidity (if any), Postoperative Complications (if any) | LR, NB CART, RF, GBDT, AdaBoost, XGBoost, LightGBM, CatBoost | XGBoost | Accuracy: 95%; Precision: 96%; Recall: 94%; F1-score: 95%; AUC: 0.98 | Yes, using interpretable classifiers |
| 10 | D2 | Chelazzi et al. (2021) (41) | To identify patients at risk for postoperative complications | Different type of surgery like Breast surgery, Dental surgery, Endocrine surgery, etc. | - | Request | 560/Tertiary care teaching hospital of Careggi (Azienda Ospedaliero-Universitaria di Careggi) | Patients comorbidity factors: Abnormal ECG (lef bundle branch block, lef ventricular hypertrophy, repolarization abnormalities, non-sinus rhythm), Untreated hypertension or hypertension not controlled by medical therapy, Previous thromboembolism, Stable or controlled angina, Previous myocardial infarction with no clinical or diagnostic evidence of residual ischemia, Compensated heart failure or previous heart failure, Diabetes mellitus, and etc. | Single Layer Feedforward Network with the training algorithm. | DEC | Average Classifcation Accuracy: 90%; Balanced Accuracy: 90.45%; Sensitivity: 88.9%; Specificity: 90.2%; PPV: 61.5%; NPV: 97.9% | No |
| 12 | D2 | Bishara et al. (2022) (5) | To develop a postoperative delirium risk prediction model | Different type of surgery like Neurological Surgery, Orthopedics Surgery, General Surgery, etc. | - | Request. | 24885(M:12276,F:12609)/Moftt-Long Hospital, Mission Bay Hospital | Demographics, Comorbidities, Nursing Assessments, Surgery Type, and other preoperative pre-operative electronic health data | NN, XGBoost, Clinician-Guided Regression, ML Hybrid Regression, AWOL-S | XGBoost | AUC-ROC: 0.851 | Yes, using XGBoost |
| 13 | D2 | Bai et al. (2020) (47) | To provide clinical data for the prevention of postoperative cerebral infarction and myocardial infarction | - | General | Request | 443(M:351,F:92)/Peking University Third Hospital | Demographic Data, Previous Medical History, Degree of Neck Vascular Stenosis, Blood Pressure at time points during the perioperative period, the Time of Occlusion, whether to Place the Shunt, and the time of Hospital Stay, whether to have Cerebral Infarction and Myocardial Infarction | SVM, DT, RF, ANN, Quadratic Discriminant Analysis, XGBoost | XGBoost | Accuracy: 94% | No |
| 14 | D2 | Ko et al. (2020) (48) | Identification of preoperative risk factors for postoperative acute kidney injury | Knee arthroplasty | General, Spinal | Unavailable | 5757(M:682,F:5075)/not reported | Preoperative serum creatinine levels, use of TXA, general anesthesia, use of RAASis, ASA class, and Gender | GBM | GBM | AUC: 0.78 | No |
| 15 | D2 | Suhre et al. (2020) (51) | Association of cannabis use with a small increase in the risk of postoperative nausea and vomiting | - | General | Available | 43633/University of Washington Medical Center, Harborview Medical Center | Age, ASA, Outpatient, Gender, Non-smoker, Prior PONV/Motion Sickness, Procedure Duration, Exposed to Nitrous Oxide, Surgery Higher Risk for Nausea, Total Number of Prophylactic Agents, PACU Opioids, Apfel Score | Bayesian Additive Regression Trees | Bayesian Additive Regression Trees | Mean Relative Risk: 1.19 | No |
| 16 | D2 | Cao et al. (2020) (49) | To explore whether serious postoperative complications of bariatric surgery recorded in a national quality registry can be predicted preoperatively | Bariatric Surgery | General | Unavailable | 44061/Scandinavian Obesity Surgery Registry | 5 continuous features (age, hemoglobin A1c, BMI, WC, and operation year) and 11 dichotomous features (Gender sleep apnea hypertension diabetes dyslipidemia dyspepsia depression musculoskeletal pain previous venous thromboembolism revisional surgery and the outcome, serious postoperative complications) | MLP, CNN, RNN | CNN | AUC: 0.57 | No |
Abbreviations: GB, gradient boosting; LR, logistic regression; NN, neural network; RF, random forest; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; BMI, body mass index; ASA, American Society of Anesthesiology; XGBoost, extreme gradient boosting; LDA, linear discriminant analysis; GAM, generalized additive model; AP, average precision; HPI, hypotension prediction Index; TWA, time-weighted average; CEA, carotid endarterectomy; GBR, gradient boosted regression; GBRT, gradient boosted regression trees; ACLR, anterior cruciate ligament reconstruction; ML, machine learning; SVM, support vector machine; AUROC, area under the receiver operating characteristics; AUPRC, area under the precision-recall curve; ICU, intensive care unit; EMR, electronic medical record; NLP, natural language processing; DT, decision tree; NB, naïve bayes; ANN, artificial neural network; GBDT, gradient boosted decision trees; ECG, electrocardiogram; ROC, receiver operating characteristics; TXA, tranexamic acid; RAASis, renin–angiotensin–aldosterone system inhibitors; GBM, gradient boosting machine; PONV, postoperative nausea and vomiting; PACU, post anesthesia care unit; WC, waist circumference; MLP, multi-layer perceptron; CNN, convolutional neural network; RNN, recurrent neural network.
| No. | SubCat. | Study | Goal | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D3 | Magunia et al. (2021) (54) | To stratify patient risk and predict ICU survival and outcomes | Request | 1039(M:853,F:333)/27 German hospitals | A total of 49 variables were used for the ML models, including: Demographic data, Past medical history, Previous medications, Current illness data, Laboratory values as well as outcome data | Explainable Boosting Machine (EBM), EBM with 10 interactions, SVC & RF | EBM with 10 interactions | Balanced Accuracy: 64%, PR-AUC: 0.81 | Yes, using interpretable model |
| 2 | D3 | Hu et al. (2021) (59) | To incorporate key variables into a parsimonious model for electroencephalographic seizure prediction in critically ill children | Unavailable | 719/Research Electronic Data Capture database | Clinical data included age, Gender, prior neurodevelopmental disorders, medications, CEEG indication, hospital and PICU admission and discharge dates, presence of clinically evident seizures prior to CEEG, acute encephalopathy category (epilepsy-related, acute structural, or acute non-structural) based on the primary presenting problems/diagnoses available at the time of admission, and mental status (comatose or not baseline or not) | RF, Least Absolute Shrinkage and Selection Operator & DL Important Features | RF | Training Accuracy: 96.3%; Validation Accuracy: 74%; AUROC: 0.706; F1-score: 73.2% | Yes, using ranking algorithm based on the relative importance |
| 3 | D3 | Cherifa et al. (2021) (56) | To predict simultaneously the Mean Arterial Pressure and the Heart Rate | Available | 22247(M:1424,F:884)/ MIMICIII waveform matched subset from the five ICUs of Boston's Beth Israel deaconess medical center | Patients characteristics (age, gender, ...), Initial severity scores (SOFA, SAPS-II), Type of intensive care unit, Treatment (sedation, vasopressors, mechanical ventilation) & Physiologic signals (pulse, oximetry, heart rate, systolic arterial pressure, mean arterial pressure and diastolic arterial pressure) | Multi-task Learning Physiological Deep Learner (MTL-PDL) & Single-task Learning Physiological Deep Learner (STL-PDL) | MTL-PDL | RMSE of MTL-PDL was less than RMSE of STL-PDL | Yes |
| 4 | D3 | Moghadam et al (2020) (57) | To predicts hypotension up to 30 min in advance based on the data from only 5 min of patient physiological history in ICU | Unavailable | 1000(M:604,F:396)/MIMIC III database | A set of 33 scalar features are used to represent each data point. At each data point, including: Arterial blood pressure, Heart rate, Systolic blood pressure, Diastolic blood pressure, Respiration rate, Peripheral capillary oxygen saturation, Pulse pressure, Mean arterial pressure, Cardiac output, MAP to HR ratio, and etc. | LR, a variety of SVM algorithms, and KNN with different kernels | LR | Accuracy: 94%, sensitivity: 85%, specificity: 96%, PPV: 81% | Yes, using feature importance |
| 5 | D3 | Cherifa et al. (2021) (58) | To predict an Acute hypotensive episodes, 10 minutes in advance | Available | 1320/MIMIC II database(1151) & External dataset from Lariboisière hospital was used for external validation(169) | Age, Gender, type of care unit, severity scores, and time-evolving characteristics such as Mechanical ventilation, vasopressors, or sedation medication as well as features extracted from physiological signals: heart rate, pulse oximetry, and arterial blood pressure | For Random partial sample: Bayesian Generalized Linear Regression, XGBoost, Gradient Boosting, Interaction LR, LR, NN, Penalized LR, RF, Recursive Partitioning, Discrete Super learner and Super Learner & For full sample: Generalized Linear Mixed algorithms via PQL, Generalized Linear Mixed algorithms via ML, Linear regression using Generalized Least Squares, Discrete Super learner and Super Learner | For the first task, that is, AHE prediction based on 1 random period per patient (random partial sample): RF & For AHE prediction based on all periods (full sample): The Generalized Linear Mixed ensemble weight of 0.70 | RF: BS: 0.086 & The Generalized Linear Mixed ensemble: 0.082 | No |
| 6 | D3 | Yun et al. (2021) (55) | To predict in-hospital death of critically ill patients with considerable accuracy and identify factors contributing to the prediction power | Request | 1384/Surgical Intensive Care Unit of their institution | Demographic variables (Age, gender, BMI, ...), Disease-specific variables (Disease diagnosis, origin, ...), Surgical variables (Type of surgery, operation name, ...), Laboratory variables (Blood gas analysis, WBC, ...) & Hemodynamic variables (Use of inotropes and use of vasopressors) | DT, NN, NB, RF and Hellinger Distance Estimates | RF | F1-score: 84%; Precision: 78%; Recall: 90%; AUC: 0.77 | Yes |
| 7 | D3 | Chang et al. (2022) (60) | To predict ICU admission of patients with Myasthenia Gravis | Request | 228/Shin-Kong Wu Ho-Su Memorial Hospital | Medical records including information on the age, Gender, age at diagnosis, disease duration, autoantibodies present, medications used, maximum dosage of corticosteroid before admission, thymic histology, history of thymectomy, treatment during hospitalization and length of ICU admission | Classification and regression tree, C4.5 & C5.0 | C5.0 DT | Accuracy Mean (SD): 94.2%; Sensitivity Mean (SD): 99.4%; Specificity Mean (SD): 63.9%; AUC Mean (SD): 0.814; F1-score Mean (SD): 96.7% | Yes |
| 8 | D3 | Hayasaka et al. (2021) (61) | To classify intubation difficulties from the patient’s facial image | Request | 202(M:92,F:110)/Yamagata University Hospital | Facial Images | Classification and regression tree, C4.5 & C5.0 | CNN | Accuracy: 80.5%; Sensitivity: 81.8%; Specificity: 83.3%; AUC: 0.864 | No |
| 9 | D3 | Wu et al. (2021) (62) | To investigate the association between culture positivity during admission and long-term outcome in critically ill surgical patients | Request | 6748/Taichung Veterans General Hospital, Taiwanese National Health Insurance Research Database | Age, Gender, BMI, Comorbidities, Severity Score, Shock, Early Fuid Overload, Receiving Mechanical Ventilation, the Need of Renal Replacement Therapy for Critical Illness | Log-rank test + multivariable Cox proportional hazards regression model | Log-rank test + Multivariable Cox proportional hazards regression model | Hazard Ratio: 1.579 | No |
| 10 | D3 | Ling et al. (2021) (63) | Investigate the relationship between the red cell distribution width and the prognosis of patients with Sepsis-associated thrombocytopenia | Request | 809(M:444,F:365)/MIMIC-III database | Age, Gender, Hypertension, Diabetes, Stroke, Heart diseases, Red Cell Distribution Width, Hemoglobin, Hematocrit, White Blood Cells, Platelet count, Prothrombin Time, Activated Partial Thromboplastin Time, Lactate, Sequential Organ Failure Assessment score | XGBoost | XGBoost | Sensitivity: 70%; Specificity: 57%; AUC: 0.646 | Yes, using SHapley Additive exPlanations |
| 11 | D3 | Sarton et al. (2021) (64) | Investigate the association between early brain MRI data and functional outcomes of patients with severe herpes simplex encephalitis at 90 days after ICU admission | Unavailable | 138(M:75,F:63)/34 ICUs in France | Patient’s history, clinical, laboratory, and brain electrophysiologic data | Multivariable LR | Multivariable LR | AUC: 0.87; Goodness of fit (Hosmer and Lemeshow test): 0.75; Accuracy: 81.4% | No |
| 12 | D3 | Elmer et al. (2020) (65) | To predict Cerebral Performance Category using longitudinal data after cardiac arrest | Unavailable | 1010(M:626,F:384)/not reported | EEG data | Group-Based Trajectory Modeling (GBTM)-unadjusted, GBTM-Ocov, GBTM-Risk, GBTM Ocov+Risk, K-means-unadjusted, K-means-Adjusted, Bayesian regression | GBTM-Risk | Sensitivity: 38.3% | Yes, using Centers of Clusters |
Abbreviations: ICU, Intensive Care Unit; ML, machine learning; EBM, explainable boosting machine; SVC, support vector classifier; RF, random forest; PR-AUC, precision recall area under the curve; CEEG, continuous electroencephalogram; PICU, Pediatric Intensive Care Unit; DL, deep learning; AUROC, area under the receiver operating characteristics; MTL-PDL, multi-task learning physiological deep learner; STL-PDL, single-task learning physiological deep learner; RMSE, root mean square error; MAP, mean arterial pressure; HR, heart rate; RR, respiratory rate; ECG, electrocardiogram; ABP, arterial blood pressure; Resp, respiration rate; SpO2, peripheral oxygen saturation; PP, pulse pressure; CO, cardiac output; LR, logistic regression; SVM, support vector machine; KNN, K-nearest neighbor; PPV, positive predictive value; XGBoost, extreme gradient boosting; NN, neural network; AHE, acute hypotensive episodes; BS, brier score; BMI, body mass index; DT, decision tree; NB, naïve bayes; AUC, area under the curve; CNN, convolutional neural network; MRI, magnetic resonance imaging; EEG, electroencephalogram; GBTM, group-based trajectory modeling.
3.5. Category E: Ultrasound Guidance
Determining the appropriate site for injecting an anesthetic drug is a significant challenge in anesthesia, particularly in regional anesthesia. Injecting the drug around the relevant nerve is essential to achieve nerve block, temporarily blocking pain signals. However, injecting the drug at the wrong site or at a long distance from the nerve can lead to dangerous complications. Anesthesiologists often face difficulty in accurately performing this task in real-time. To address this challenge, AI techniques, particularly image processing, have been employed to induce regional anesthesia under ultrasound guidance. These techniques allow physicians to visualize the internal structure of organs and determine the correct injection site more easily.
The reviewed studies in this category can be divided into two subcategories based on the AI algorithms used for determining the appropriate injection site: DL-based algorithms and tracking algorithms based on correlation filters.
Most of the articles in this category fall into the first subcategory. In one study (66), a novel algorithm was proposed for accurate needle tip placement under ultrasound guidance when the needle body is invisible and the tip has low intensity. The algorithm first extracts the needle tip properties in successive ultrasound frames using a detection scheme and then predicts the location of the needle tip using a deep neural network consisting of CNN and LSTM recurrent units. The study achieves an error rate of 0.06 ± 0.02 mm for the needle entry point and a processing time of 0.064 seconds. However, the limitations included using ex vivo data and specific needle types.
In another study (67), the DL model was used to determine the anesthesia site by dividing the patients into control and algorithm groups. The algorithm group used ultrasound guidance and a deep CNN SegNet (68) to determine the anesthesia site, leading to significant improvements in the average injection duration and needle insertion depth, compared to the control group.
In another study (69), a preliminary assessment of an AI system was performed using a deep CNN network for semantic segmentation of ultrasound images. The aforementioned study focused on seven specific nerve blocks, and the proposed model aimed to detect the presence of these seven nerve blocks in the input images.
Studies in the second subgroup focus on tracking arteries instead of nerves in ultrasound images due to the low quality of the images, making nerve detection difficult. In one study (70), real-time tracking models were designed using a modified kernelized correlation filter (KCF) and modified discriminative correlation filter with channel and spatial reliability method (CSR-DST). The CSR-DST algorithm performed faster; however, the KFC provided better results and was identified as the superior algorithm. Table 8 shows the key characteristics of the reviewed studies in this category.
| No. | Study | Goal | Type of Anesthesia | Enhancement Filter(s) | Nerve Block(s) | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Paris and Hafiane (2021) (70) | To track arteries in ultrasound guidance to find a proper place to inject the anesthetic drugs | Regional | Kernelized Correlation filter, Discriminative Correlation filter | - | Unavailable | 71/not reported | Fd is introduced as features that are extracted from images after applying the kernels. | Gradient descent applied to the search for ellipses, Modified KCF, Modified CSR-DST. | Modified CSR-DST | Mean Error: 15.16 STD Error: 25.51 FPS: 63.06 Precision ~ 95% |
| 2 | Bowness et al. (2021) (69) | To perform semantic segmentation of the input ultrasound videos | Regional | - | Supraclavicular level brachial plexus: Subclavian artery, brachial plexus nerves, first rib, pleura. Erector spinae plane (thoracic region): Trapezius/rhomboid/erector spinae (group) muscles, vertebral transverse process/rib, pleura. Rectus sheath: Rectus abdominis muscle, rectus sheath, peritoneal contents. Adductor canal: Femoral artery, saphenous nerve, sartorius/adductor longus, femur | Unavailable | 144 & 244/The Royal Gwent Hospital, Ystrad Mynach Hospital, StWoolos Hospital & Nevill Hall Hospital | Extracted features from Deep CNN | Deep CNN Based on U-Net | Deep CNN Based on U-Net | Using statistical analysis, the Kruskal–Wallis H-test |
| 3 | Liu and Cheng (2021) (67) | To locate the anesthesia point of patients during scapular fracture surgery treated with the regional nerve block | Regional | Gaussian low-frequency filters | Scapula Regional Nerve Block | Request | 100/Jiangxi Armed Police Corps Hospital | Ultrasound Images of the Scapula of the Patients | SegNet (A brand-new deep fully CNN) | SegNet | Injection Time: 7.7 ± 2.1 min Distance between the Puncture Point and the Scapula: 62.5 ± 7.2 mm |
| 4 | Mwikirize et al. (2021) (66) | Needle tip localization during challenging ultrasound-guided insertions when the shaft may be invisible, and the tip has a low-intensity | Regional | - | - | Unavailable | 80/SonixGPS & Clarius C3 | Enhanced Tip Images and B-Mode Images | DNN(CNN+LSTM) | DNN(CNN+LSTM) | Tip Localization Error: 0.52 ± 0.06 mm Overall Computation Time: 0.064 s |
Abbreviations: KCF, kernelized correlation filter; CSR-DST, discriminative correlation filter with channel and spatial reliability method; FPS, frame per second; CNN, convolutional neural network; DNN, Deep Neural Network; LSTM, long short-term memory.
3.6. Category F: Operating Room Logistics
The studies conducted in this category focused on organizing and coordinating the affairs within the operating room. Some of the studies in this category aimed to predict the duration of each operation (71, 72); however, others focused on addressing challenges that lead to the wastage of hospital facilities and resources (73). One significant challenge is day-of-surgery cancellation (DoSC), which can be problematic for hospital staff, patients, and their families, in addition to being costly and time-consuming. To address this issue, a study (73) analyzed the electronic file information of approximately 88 000 patients, considering various variables, including economic and social factors. The study utilized several ML algorithms to understand the reasons behind the DoSC.
In two other studies conducted by Gabriel et al. (71) and Jiao et al. (72), ML algorithms were used to predict the end time of surgery. Additionally, in Gabriel’s study (71), predicting the patient's recovery period was another goal. All studies in this category utilized AI algorithms, particularly ML, to optimize hospital facilities and staff management. Table 9 shows further detailed information about the reviewed studies in this category.
| No. | Study | Goal | Type of Surgery | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gabriel et al. (2022) (71) | To predict the following composite outcome: 1. surgery finished by the end of the operating room block time and 2. the patient was discharged by the end of the recovery room nursing shift. | Outpatient surgery | Unavailable | 13447/not reported | The surgical procedure, surgeon identification, ASA score, age, Gender, weight, surgical service line, scheduled surgical incision time, scheduled room time, actual room time, actual PACU length of stay | LR, RF Classifier, Balanced RF, Balanced Bagging, Simplefeedforward NN & SVM classifier | Balanced Bagging (Using SMOTE) | Precision: 83%; Recall: 77%; Matthew’s correlation coefficient: 0.642; Sensitivity: 77.3%; Specificity: 87.1%; AUC: 0.905 | Yes, the feature importance graph based on the balanced bagging approach |
| 2 | Jiao et al. (2020) (72) | To predict a continuous probability distribution of surgical case durations | Various surgical services | Unavailable | 52735/Central operating location at St. Louis Children’s Hospital, a free-standing, tertiary-care, pediatric hospital | Categorical (ASA, inpatient status, day of week), Continuous (scheduled surgery duration, patient age), Unstructured text (procedure name, surgical diagnosis) variables | A Neural Network (Mixture Density Network (MDN)), Tree-based methods (DT, RF, and GBT), non-ML statistical method (Bayesian statistical method) | MDN | Continuous Ranked Probability Score: 18.1 minutes | Yes, permutation importance was calculated for the MDN |
| 3 | Liu et al. (2021) (73) | To understand potential underlying contributors to disparities in DoSC rates across neighborhoods | - | Unavailable | 88013/Cincinnati Children’s Hospital Medical Center and Texas Children’s Hospital | All features were in one of these categories: Transportation, Preoperative phone calls, Recent healthcare use, Prior cancellation behaviors, Surgery-related factors | Non-spatial regression models (GLM, L2-normalized GLM, SVM with polynomial kernels and DT, Spatial regression models (SAR model, spatial Durbin model, SEM, spatial Durbin error model, spatial moving average, and SAR confused models), CNNs & Graph Convolutional Networks | An L2-normalized generalized LR model | RMSE: 0.01305, 95% CI: 0.01257-0.01352 | Yes, using feature importance generated from the best-performing L2-normalized generalized LR model |
Abbreviations: ASA, American Society of Anesthesiology; PACU, Post Anesthesia Care Unit; LR, logistic regression; RF, random forest; NN, neural network; SVM, support vector machine; SMOTE, synthetic minority oversampling technique; AUC, area under the curve; MDN, mixture density network; DT, decision tree; GBT, gradient boosting-based tree; ML, machine learning; DoSC, day-of-surgery cancellation; GLM, generalized linear model; SAR, spatial autoregressive; SEM, spatial error model; CNN, convolutional neural network; RMSE, root mean square error.
3.7. Category G: Depth of Anesthesia
The anesthesia process consists of three stages: Anesthesia induction, maintenance of anesthesia, and recovery. In the anesthesia induction phase, the patient enters the initial phase of anesthesia when a specialist physician injects induction drugs, either through injection or inhalation. During the maintenance phase of anesthesia, the patient is maintained at an appropriate depth of anesthesia by administering the proper dose of maintenance medication. In the last stage, the patient recovers from anesthesia as the drugs are metabolized and eliminated from the body. Throughout these stages, the injection of relevant drugs by the anesthesiologist requires accurate knowledge and information about the depth of anesthesia and the patient's level of consciousness. Measuring the patient's physiological and clinical criteria simultaneously to assess the depth of anesthesia is challenging for physicians and prone to human errors. Artificial intelligence techniques can be employed to reduce these errors and improve performance in categorizing and monitoring the depth of anesthesia.
The studies in this category are divided into three groups based on the type of data used in each study. These groups include studies based on EEG signals, physiological-clinical variables, and the combination of EEG signals and physiological-clinical variables.
The brain is the main human organ and the first area to be affected after injecting anesthetic drugs. Due to the good reflection of brain activity in EEG signals, they are used as supplement monitoring to determine the level of consciousness more accurately.
In studies based on EEG signals, researchers have developed monitoring systems using EEG-based criteria to evaluate the depth of anesthesia more accurately (3, 74-88). The bispectral index (BIS) is a common diagnostic index used to measure the depth of anesthesia based on EEG signals. In one study (75), a combined DL structure was proposed, consisting of three networks: CNNs using an inception module, LSTM, and one attention layer. The regression model’s output was a BIS index used to determine the patient's depth of anesthesia, achieving 88.71% accuracy.
In other studies, new indices or improved versions of the previous indices were defined to determine the depth of anesthesia and enhance monitoring (78, 84). For instance, the Poincaré index was introduced to target a specific frequency range of 20 to 30 Hz, and it was combined with the classical Poincaré 0.5 - 47 Hz index using DL-improved anesthesia depth monitoring (84).
Using EEG-based indices as complementary monitoring can offer various benefits in assessing the depth of anesthesia and the patient's level of consciousness. However, there are certain limitations associated with EEG, such as low performance with volatile anesthetics, long latency, and susceptibility to interference from surgical stimulation. Apart from EEG-based studies, other data types have been used to train models and determine the level of consciousness in articles in this category (89-91). Dubost et al. (89) and Zhan et al. (90) utilized physiological or clinical and functional magnetic resonance imaging (fMRI) data as alternatives to EEG signals. Various methods, such as hidden Markov models and deep neural models, were employed as learning models in these studies (89, 90).
In the third group of studies, researchers combined EEG signals with other signals, such as auditory evoked potentials (AEP), to determine the level of consciousness by creating a new index (92, 93). In another study (93), several classification algorithms in ML were utilized to construct the unified index, with each model trained using EEG signal parameters as features. The support vector machine (SVM) model exhibited the best performance in this study, achieving a prediction probability of 0.935. The reviewed studies in this category are summarized in Table 10.
| No. | Study | Goal | Type of Anesthesia | Induction Drug(s) | Depth of Anesthesia Levels | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abel et al. (2021) (74) | To construct classification models for real-time tracking of anesthesia unconscious state during anesthesia | General | Propofol & Sevoflurane | Consciousness & Unconsciousness | Request | Not reported/ Massachusetts General Hospital | The feature sets used were the multi-tapered EEG spectral power, EEG bendwise power, the first three principal component scores of the multitaper spectrogram, the linear discriminant score of the multitaper spectrogram (LDA, with supervised learning performed by including the labels), and the first ten principal component scores of a set of features generated by a deep CNN. | Use the below algorithms in 3 ways: Without HMM, with 2-State-HMM, and With 6-State-HMM. The algorithms are Multitaper Spectrogram, BWP, PCA, LDA, and CNN. | LDA+HMM2 | AUC ~ 0.99 |
| 2 | Dubost et al. (2021) (89) | To predict and assess states based on four physiological variables: Heart Rate, Mean Blood Pressure, Respiratory Rate, AA Inspiratory Concentration | General | Propofol & Ketamine | Awake, The Loss of Consciousness (LOC), The anesthesia, The Recovery of Consciousness (ROC), Emergence. | Unavailable | 30 (M: 20, F: 10)/Begin military teaching hospital | Heart Rate, Systolic arterial blood pressure, Diastolic arterial blood pressure, Mean arterial blood pressure, Saturated percentage of dioxygen, End-tidal carbon dioxide, Anesthesia agent, AA expiratory concentration, AA inspiratory concentration, Total minimum alveolar concentration, Fraction inspired of dioxygen, Mean alveolar concentration, Fraction inspired nitrous oxide, End-tidal nitrous oxide, Respiratory rate, BIS, BIS burst suppression ratio, BIS electromyography & Demographic Features (Age, Gender, etc) | HMM | HMM | Error Prediction: 0.18 |
| 3 | Afshar et al. (2021) (75) | To get EEG signals and continuously predict the BIS | General with few cases receiving Sedation/ Analgesia and Local anesthesia | Propofol and/or Remifentanil | Deep Anesthesia (DA, BIS: 0-40), General Anesthesia (GA, BIS: 40-60), Light Sedation (S, BIS: 60-80) & Awake (W, BIS: 80-100) | Unavailable | 176 (M: 102, F: 74)/Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, College of Medicine | Extracted features from EEG signals by DNN age, height, weight, and anesthesia duration | Combinatorial DL structure involving CNN (inspired by the Inception module), Bidirectional LSTM, an Attention Layer | Combinatorial DL structure | AUC: 0.811 ± 0.527, sensitivity: 77.62%, accuracy: 88.71% |
| 4 | Zhan et al. (2021) (90) | To distinguish different anesthesia states, providing a secondary tool for DoA assessment | General | Intravenous Midazolam, Propofol, Sufentanil & Cisatracurium | Anesthesia Induction, Anesthesia Maintenance, Anesthesia Recovery | Request | 23/Second Affiliated Hospital of the Army Medical University | Four Heart Rate Variability-derived features in the time and frequency domain were extracted from an electrocardiogram, including HRV high-frequency power, Low-frequency power, High-to-low-frequency power ratio, and Sample entropy and age, Height, Weight, BMI, Duration of surgery, Anesthetic management, Maintenance drugs infusion rate, Additional drugs administrated when approaching the end of surgery. | LR, SVM, DT & DNN | DNN | Precision of anesthesia induction: 58.1%, recall of anesthesia induction: 88.1%, precision of anesthesia maintenance: 96%, recall of anesthesia maintenance: 94.7%, precision of anesthesia recovery: 56.6%, recall of anesthesia recovery: 57.8%, classification accuracy: 90.1% |
| 5 | Duclos et al. (2021) (76) | To classify different states of AEC and wPLI measure of FC | - | Propofol | Baseline, Light Sedation, Unconscious, Pre-ROC & Recovery | Request | 9/not reported | Extracted features from Functional Connectivity time-series signals by AEC & wPLI | Use the below algorithms in 3 ways: With AEC Features, with wPLI Features, and with both AEC and wPLI Features. The algorithms are Linear kernel SVM, RBF kernel SVM, LDA | Linear kernel SVM (C=0.1) with AEC Feature for Unconscious class | Accuracy ~ 85% |
| 6 | Avilov et al. (2021) (77) | Detection of intraoperative awareness during general anesthesia, especially | General | Propofol | - | Unavailable | 22 (M: 10, F: 12)/Inria | Extracted features from EEG signal by CSP filters, Riemannian geometry, linear discriminant analysis | CSP+LDA, Minimal Distance to the Riemannian Mean, Tangent Space+LR, DeepConvNet, ShallowConvNet, EEGNet-2.32 & EEG-4.8 | EEGNet-4.8 with 128 Electrodes | Accuracy: 94.5%, false-positive rate: 6.1% |
| 7 | Sook Ra et al. (2021) (78) | Develop a new DoA index for monitoring the DoA | - | Propofol | Consciousness, Light Anesthesia, Deep Anesthesia | Request | Not reported/University of Southern Queensland | Extracted features from EEG signals by entropy methods (SE and PE) and age, Weight, Height, Gender, Midazolam, Alfentanil, Propofol, Parecoxib, Fentanyl | SVM, DL Algorithm & NN, LR | LR | The Pearson correlation coefficient, RMSE, and execution time of LR were the best. |
| 8 | Sanz Perl et al. (2021) (92) | To develop whole-brain computational models to show that the stability of conscious states provides information complementary to their similarity to conscious wakefulness | - | Propofol | Sleep, Propofol Anesthesia & Post-Comatose Disorders of Consciousness | Part of the Dataset (Sleep data) is available | 43 (M: 27, F: 16)/Medical School of the University of Liège | Extracted features from fMRI and EEG data & Gender, Age at Scan, Etiology, Days since Injury, Aud. Function, Vis. Function, Mot. Function, Oro/Ver Function, Communication, Aurosal, #CRS-R assessment | RF | RF | RF has good performance in this study. |
| 9 | Madanu et al. (2021) (3) | To extract features from EEG signals to predict DoA | General | Propofol | Anesthesia Deep, Anesthesia OK, Anesthesia Light | Unavailable | 50/National Taiwan University Hospital | EEG data | CNN with 5, 6 & 10 layers, AlexNet, Pre-Trained VGG16, Pre-trained VGG19, Pre-trained InceptionRESV2 | CNN with 10 layers | Accuracy: 83% |
| 10 | Fali Li et al. (2021) (79) | To detect the time onset at which patients lost their consciousness within the duration of (10, 61) after being injected with Propofol | General | Propofol | The Loss of Consciousness (LOC), Resting | Unavailable | 30/Shanghai Sixth People’s Hospital | Features extracted from EEG signals time-series by multi-channel cross-fuzzy entropy | Corresponding time-varying cross-fuzzy networks(C-FuzzyEn) time-varying coherence networks | C-FuzzyEn | The Long-Range Connectivity (LRC) of C-FuzzyEn is better than the LRC of COH (LRC is a parameter that measures the number of frontal-occipital connectivity) |
| 11 | Yuqin Li et al. (2021) (80) | To track the loss of consciousness and recovery of consciousness under General Anesthesia, using EEG signals | General | Propofol | Anesthesia Induction (i.e., LOC), Anesthesia Recovery (i.e., ROC), the Resting State (i.e., Resting) | Request | 30 (M: 14, F: 16)/Shanghai Sixth People's Hospital | Three Feature sets, including Spatial Pattern of the Network features, Properties & SPN features + Properties | SVM | SVM | Accuracy: 95%, sensitivity: 93.33%, specificity: 96.67% |
| 12 | Tacke et al. (2020) (93) | Construct a combined electroencephalographic anesthesia index that predicts responsiveness in anesthetized patients. | General | Remifentanil, Sevoflurane, Propofol & Succinylcholine | - | Unavailable | 39/not reported | EEG Parameters: Weighted Spectral Median Frequency, quotient of WSMF, Spectral Entropy, Hurst Exponent, Approximate Entropy, Lempel-Ziv Complexity, Permutation entropy Auditory Evoked Potentials Parameters: Wavelet Coefficients, Amplitudes and latencies of Wavelet Coefficients, Signal Energies based on Wavelet Coefficients, Maximum Amplitude of Retransformed AEPs, Variance of the Second Derivative of Wavelet Coefficients | SVM, NB Classifier, LR, MLP, Bayesian Net, J48 | SVM | Pk: 0.935±0.11 |
| 13 | Campbell et al. (2020) (91) | Identifying degrees of pathological unconsciousness in clinical patients under anesthesia via resting-state fMRI | General | Propofol, Remifentanil & Succinylcholine | Anesthesia-SHH: Awake, Light Sedation, Deep Sedation or General Anesthesia Anesthesia-WI: Wakefulness Baseline, Light Sedation, Deep Sedation, Recovery DOC: Healthy, Unresponsive Wakefulness Syndrome, Minimally Conscious State | Available | 93/Anesthesia-SHH, Anesthesia-WI, DOC | Resting-State fMRI Feature Data: 32 features, including Amplitude of Low-Frequency Fluctuations (3), Within Network Functional Connectivity FC(8), Between Network Functional Connectivity FC(21) | SVM, Extra Trees, ANN | All | AUC>0.95 |
| 14 | Ramaswamy et al. (2020) (81) | Estimate the depth of sedation via frontal EEG signals | - | Propofol, Dexmedetomidine, Sevofurane & Remifentanil | Awake, Sedated | Unavailable | 66/Using a 16 channel Neuroscan® EEG monitor (Compumedics USA, Limited, Charlotte) | EEG Signals: Nonlinear energy operator, Activity, Mobility, Complexity, Root Mean Square Amplitude, Kurtosis, Skewness, Mean of Amplitude Modulation, Standard Deviation of AM, Skewness of AM, Kurtosis of AM, Burst Suppression ratio/min, Pδ=mean power in delta band, Pθ=mean power in theta band, Pα=mean power in alpha band, Pσ=mean power in spindle band, Pβ=power in beta band, PT=total spectral power, Pδ/PT, Pθ/PT, Pα/PT, Pσ/PT, Pβ/PT, Pδ/Pθ, Pα/Pθ, Pσ/Pθ, Pβ/Pθ, Pα/Pθ, Pσ/Pθ, Pβ/Pθ, Mean of Frequency Modulation, Standard Deviation of FM, and extra. | Elastic Net LR, SVM with Gaussian Kernel, RF, Ensemble Tree with Bagging | Ensemble Tree with Bagging | AUC: 0.88 |
| 15 | Kashkooli et al. (2020) (82) | Design drug-specific models to improve the performance of automated anesthetic state monitors | General | Sevoflurane & Ketamine | Awake, Sedation, General Anesthesia | Unavailable | 12 (M: 7, F: 5)/Waveguard system with a standard EEG cap(64 channels, ANT Neuro) | EEG data: Mean Power of Slow, Mean Power of Theta, Mean Power of Low-Beta, Mean Instantaneous Frequency, Kurtosis of Instantaneous Frequency, Hjorth Mobility, Permutation Entropy, Higuchi Fractal Dimension | KNN | KNN | F1-score: 94% |
| 16 | Lee et al. (2020) (83) | To identify brain states independent of the actual anesthetic concentration | - | Desflurane | - | Unavailable | 7 Rats (M: 7, F: 0)/SmartBox (NeuroNexus Technologies) | Spike Rate, Local Variation, Total Number of Spikes, Longest Period Below Mean, Sample Entropy | Hierarchical Agglomerative Algorithm with Ward’s Linkage Method | Hierarchical Agglomerative Algorithm with Ward’s Linkage Method | - |
| 17 | Hayase et al. (2020) (84) | Improve anesthesia depth monitoring using the 20-Hz to 30-Hz hierarchical Poincaré analysis. | General, Local | Propofol, Sevofurane, Remifentanil & Fentanyl | Lighter Anesthesia, Deeper Anesthesia | Unavailable | 30 (M: 16, F: 14)/Kyoto Chubu Medical Center | Poincaré-index20–30 Hz, Poincaré-index0.5–47 Hz, Electromyogram EMG70–110 Hz, Suppression Ratio | MLPNN | MLPNN | Correlation Coefficient: 0.87 RMSE: 7.09 |
| 18 | Shalbaf et al. (2020) (85) | To assess the level of hypnosis with Sevoflurane | - | Sevoflurane | Awake, Light Anesthesia, General Anesthesia, Deep Anesthesia | Unavailable | 17/not reported | EEG data: Frequency Index (Beta), Sample Entropy, Shannon Permutation Entropy, Detrended Fluctuation Analysis | SVM | SVM | Accuracy: 94.11% |
| 19 | Park et al. (2020) (86) | To present a real-time EEG-based DoA monitoring system | General | Sevoflurane & Propofol | - | Available | 374/VitalDB constructed at Seoul National University Hospital | EEG data | ANESNET | ANESNET | MSE: 0.048 MAE: 0.05 Pearson Correlation Coefficient: 0.676 Concordance Correlation Coefficient: 0.566 |
| 20 | Li et al. (2020) (87) | To monitor DoA | - | Sevoflurane | - | Request | 20/Waikato Hospital in Hamilton | EEG data | LSTM and Sparse Denoising Autoencoder | LSTM and Sparse Denoising Autoencoder | P_k: 0.8556±0.0762 |
| 21 | Ihalainen et al. (2021) (88) | Evaluate the evidence for the posterior hot zone theory of consciousness by modeling the relative contributions of three resting-state networks for Propofol-induced loss of consciousness. | General | Propofol | Behavioural Responsiveness, Sedation, Loss of Consciousness with Clinical Unconsciousness, Recovery of Consciousness | Request | 10 (M: 4, F: 6)/Faculty of Medicine of the University of Liège | EEG data | Dynamic Causal Modelling (DCM) (Combination of 3 Networks: Default Mode Network (DMN), SAlience Network (SAN), Central Executive Network (CEN)) | In Frontoparietal Connections: DMN In Frontal Connections: SAN In Parietal Connections: SAN All Connections: Combination of 3 Networks | AUC: 0.78, accuracy: 80%, mean posterior probabilities: 0.67, recall: 78% |
Abbreviations: EEG, electroencephalogram; LDA, linear discriminant analysis; CNN, convolutional neural network; HMM, hidden markov model; BWP, bandwise power; PCA, principal component analysis; AUC, area under the curve; AA, anesthesia agent; BIS, bispectral index; ASA, American Society of Anesthesiology; DNN, deep neural network; DL, deep learning; LSTM, long short-term memory; DoA, depth of anesthesia; BMI, body mass index; LR, logistic regression; SVM, support vector machine; DT, decision tree; AEC, amplitude envelope correlation; wPLI, weighted phase lag index; FC, functional connectivity; RBF, radial basis function; CSP, common spatial pattern; NN, neural network; RMSE, root mean square error; RF, random forest; SPN, spatial pattern of the network; WSMF, weighted spectral median frequency; AEPs, auditory evoked potentials; NB, naïve bayes; MLP, multi-layer perceptron; P_k, prediction probability; DOC, disorders of consciousness; ANN, artificial neural network; AM, amplitude modulation; FM, frequency modulation; KNN, K-nearest neighbor; MLPNN, multi-layer perceptron neural network; MSE, mean square error; MAE, mean absolute error; DMN, default mode network; SAN, SAlience network.
3.8. Category H: Control of Anesthesia Delivery
Managing the level of a patient’s anesthesia by considering the appropriate dose of an anesthetic drug is a critical goal in the control of anesthesia delivery. Studies in this field focus on designing models to keep patients at certain levels of anesthesia or suggest measures to address challenges faced by anesthesiologists in this area. Making critical decisions about the patient's condition, such as determining the proper dose of an injectable anesthetic drug and controlling the patient's consciousness level by considering several parameters, is considered one of the most critical challenges for anesthesiologists during surgery. Some studies indicated that AI models could play an influential role in providing decision support for anesthesia delivery. Artificial intelligence algorithms can be utilized to determine the appropriate dose of an anesthetic drug for each patient, aiming to achieve the desired level of anesthesia.
In this category, many of the reviewed studies aim to determine or predict the appropriate dose of anesthetic drugs to achieve the desired level of anesthesia. For instance, Ingrande et al. (94) compared two biological models and a gated recurrent unit (GRU) network, where the GRU model demonstrated superior performance. Another study (95) designed a model to predict whether the patient will need remifentanil in the next n minutes using ML algorithms, such as SVM and the LSTM network, with LSTM being identified as the preferred model. Systolic blood pressure (SBP) was identified as the most important feature using the Shapley interpretability technique. In another study, Wei et al. (96) developed a decision tree model to determine the appropriate dose of local anesthetic hyperbaric bupivacaine during a cesarean section. The interpretability of the decision tree and the possibility of analyzing the results were among the advantages of this study.
Furthermore, Schamberg et al. (97) proposed a model for determining the appropriate dose of propofol using deep reinforcement learning based on the pain, sedation, and intensity (PSI) index. Sharma et al. (98) designed an optimal controller using type-2 fuzzy logic to determine the appropriate dose of sodium nitroprusside to maintain the patient's mean arterial pressure (MAP) at an appropriate level.
By reviewing the studies focusing on determining or predicting the appropriate dose of anesthetic drugs, it is evident that using deep recurrent neural networks, such as LSTM and GRU, and applying interpretable methods to explain the output of these networks lead to desirable outcomes, particularly when dealing with time series data.
Another critical issue in the discussion of anesthesia delivery control is the need for the physician to be informed of the drug concentration level in the patient’s blood to determine the appropriate drug dose. Due to the challenges of performing complex calculations under operating conditions, devices have been designed and built to calculate the relevant drug concentration and report it to the physician. For instance, a study (99) used a model based on a support vector regression algorithm to compensate for errors in drug concentration measurements due to continuous sensor exposure to propofol, which might lead to sediment formation and inaccurate reporting of drug concentrations to the physician. Table 11 shows further details about each reviewed study in this category.
| No. | Study | Goal | Type of Anesthesia | Induction Drug(s) | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance | Interpretable? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ingrande et al. (2020) (94) | To predict the dose of propofol anesthetic during surgery | General | Propofol | Unavailable | 24 (M: 6, F: 18)/not reported | Gender, Age, Lean Body Weight, Total Body Weight, BMI, Cardiac Output | 4-compartment Model, Recirculatory Model, GRU | GRU | MPE: 0.161 MSE: 20.83 | No |
| 2 | Sharma et al. (2020) (98) | To improve the drug infusion to the automatic control of mean arterial blood pressure for maintaining the mean arterial pressure to 100 mmHg | General | Sodium & Nitroprusside | Unavailable | Not reported/not reported | - | Type-2 fuzzy logic, Cuckoo Algorithm (Optimization) | Type-2 fuzzy logic, Cuckoo Algorithm | Error ≈ 0 | No |
| 3 | Miyaguchi et al. (2021) (95) | To determine whether Remifentanil will be given to the patient in the next n minutes. | General | Remifentanil | Unavailable | 210 (M: 103, F: 107)/Okayama University Hospital | Static Features: Patient Information (age, weight, height, gender) & Dynamic Features: Vital Records (HR, SBP, DBP, MAP, RR, SpO2, ETCO2) and Drug Records (Remifentanil flow) | SVM, LR, RF, ANN, LightGBM, LSTM | LSTM | Accuracy: 73%, sensitivity: 65%, specificity: 73%, precision: 2.3%, AUC: 0.75 | Yes, using Shapley |
| 4 | Aiassa et al. (2021) (99) | Build a learning model for electrochemical sensing, which compensates for the fouling effect of propofol. | General | Propofol | Unavailable | 480/not reported | Using 4 chemical feature sets including 1. ip, Ep, 2. ip, Ep, nmeas, 3. ip, Ep, Q, 4. ip, Ep, Q, nmeas | SVC with different kernels (Linear, polynomial, RBF, and sigmoid) & C=10 for all models | SVC (RBF) | Accuracy: 95% | No |
| 5 | Wei et al. (2021) (96) | To determine the appropriate dose of hyperbaric bupivacaine based on physical variables during cesarean section in the next 10 minutes | Neuraxial | Hyperbaric bupivacaine | Request | 684/Ethical Committee of Jiaxing Maternity | Parturient demographic Features: Age, Weight, Height, Fundal height, Demographic Features: Vertebral column length, Abdominal girth, Fetal biparietal diameter, Fetal weight, Bupivacaine dosage | DT with different hyperparameters | DT (λ Value = 0.2) | MSE: 0.084 | Yes, using Decision Tree Rules |
| 6 | Schamberg et al. (2022) (97) | To suggest the appropriate dose of anesthetic drug to automatically control the level of anesthesia during surgery | General | Propofol | Unavailable | 9/not reported | Level of Unconsciousness (LoU) error, predicted effect-site concentration, LoU change, LoU target. | DRL | DRL | Performance Error: 0.011±0.005 | Yes, using Shapley additive explanations |
Abbreviations: BMI, Body Mass Index; GRU, Gated Recurrent Unit; MPE, mean percentage error; MSE, mean square error; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; RR, respiratory rate; SpO2, peripheral oxygen saturation; ETCO2, end-tidal carbon dioxide; SVM, support vector machine; LR, logistic regression; RF, random forest; ANN, artificial neural network; LightGBM, light gradient boosting machine; LSTM, long short-term memory; AUC, area under the curve; SVC, support vector classifier; RBF, radial basis function; DT, decision tree; LoU, level of unconsciousness; DRL, deep reinforcement learning.
3.9. Category I: Monitoring
Monitoring involves the continuous assessment of a patient's hemodynamic status, including their cardiovascular and cerebral condition, in the operating room or the ICU, using specialized devices. Monitoring plays a crucial role in improving patient outcomes and the success of surgeries by maintaining vital signs within appropriate physiological ranges and quickly diagnosing and treating side effects before they lead to long-term complications. Therefore, designing highly accurate monitoring devices using AI and ML algorithms is necessary.
Artificial intelligence has been applied to monitor various aspects of a patient’s condition. For example, blood pressure monitoring during anesthesia was evaluated using ML models, such as lasso restrictive logistic regression, neural networks, and SVM (100). The SVM model demonstrated superior performance based on the Kappa criterion, which measures the classifier's conformity in classifying samples.
False alarms from ICU vital signs monitors can be common, with rates ranging from 0.72 to 0.99. Machine learning models have been utilized to reduce the frequency of false alarms, and one study included missing sensor values in the input data of the model (101).
In the neurological critical care unit (NCCU), Unal et al. (102) used statistical techniques to determine the prevalence, types, and determinants of alarms. Liu et al. (103) conducted a study in 2020 on armpit temperature monitoring using an AI-enabled wireless, non-invasive armpit thermometer called iThermonitor. This thermometer provided accurate body temperature readings, compared to mercury thermometers. Additionally, a study (104) considered the validation of clinically relevant values of the relevant compensatory reserve measurement device with a dashboard view using a simple color code to diagnose bleeding. Table 12 shows key details of the reviewed studies in this category.
| No. | Study | Goal | Type of Surgery | Dataset Availability | Number of Case/Dataset | Feature(s) | Algorithm(s) | Winner Algorithm | Winner Algorithm Performance |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pasma et al. (2021) (100) | Automated artifact removal in anesthesia blood pressure data | Non-cardiac and non-thoracic | Available | 88(M:39,F:49)/University Medical Center Utrecht | Feature Types: Systolic blood pressure, diastolic blood pressure, mean blood pressure, heart rate, pulse pressure (systolic–diastolic blood pressure), ratios between heartrate and blood pressure (systolic blood pressure divided by heartrate and diastolic blood pressure divided by heartrate), ratio between systolic and mean arterial blood pressure, and ratio between mean and diastolic arterial blood pressure | Lasso Restrictive LR, NN, SVM | SVM | Kappa: from 0.524 to 0.651 |
| 2 | Hever et al. (2020) (101) | To analyze in real-time missing sensor data to minimize false alarm rate | - | Available | 481(M:325,F:156)/Shanghai Jiao Tong University School of Medicine affiliated Ruijin Hospital | Age, Gender, NC, WC, BMI and faciocervical measurements (maximum interincisal distance (MID)), height to thyrosternum distance (H/TSD)) | SABIHC2 (It is a machine learning model based on SVM) & STOP-BANG (It is one of the most widely used questionnaires) | SABIHC2 | AUC: 0.832; Sensitivity: 91.6%; Specificity: 74.9% |
Abbreviations: LR, logistic regression; NN, neural network; SVM, support vector machine; NC, neck circumference; WC, waist circumference; BMI, body mass index; AUC, area under the curve.
4. Discussion
This review study aimed to investigate the role of AI in anesthesia while also exploring the challenges, limitations, and opportunities in this field. It emphasizes the importance of having a realistic approach and appropriate expectations toward AI technologies in improving the treatment process.
Setting realistic expectations from AI techniques ensures that outcomes are well-defined and achievable, avoiding disappointment and vague results in their use. To facilitate an organized and coherent review, the selected studies were categorized into nine groups. Among these categories, event prediction and depth of anesthesia are the largest, with 43 and 21 articles, respectively.
The current review highlights the significant growth in the number of articles on event prediction in recent years, indicating the expanding research in this area. For example, predicting hypotension during anesthesia using ML models is a prominent topic in the perioperative subcategory. On the other hand, the depth of anesthesia category has a long history of research and offers various opportunities for further investigations, particularly in determining the depth of anesthesia using EEG signals and indices, such as BIS, through deep neural networks. However, certain categories, such as control of mechanical ventilation and weaning and operating room logistics, have limited studies and are considered emerging fields in AI applications for anesthesia.
One of the challenges revealed during the review is the difficulty of comparing studies in a similar field, mainly due to variations in research datasets and evaluation criteria. Privacy concerns in medical datasets often hinder accessibility and comparability. Researchers are encouraged to use publicly available datasets or release versions of the dataset while preserving individuals' privacy to facilitate further studies, result reproduction, and problem-solving. Moreover, using multiple evaluation criteria in research studies increases comparability and the validity of systematic reviews and meta-analyses.
The interpretability of ML and DL models is essential in informing the treatment team about prediction methods, boosting confidence in the models. For instance, in the ultrasound guidance category, where CNN is commonly used with image data, interpretability can be achieved through methods, such as Shapley and local interpretable model-agnostic explanations (LIME). Researchers should also employ feature ranking techniques to identify the most influential features in predictions for various ML models.
4.1. Conclusions
Using AI as a rapidly advancing technology can have a significant impact on various fields, including anesthesiology. This review highlights the role of AI models in establishing monitoring and decision support systems in the domain of anesthesia. The studies reviewed were categorized into nine distinct areas, and the materials in each study and category were presented in an organized and tabular manner. Each section also included suggestions for future work and ideas.
The continuous progress in AI techniques offers great potential to support anesthesiologists in enhancing their performance. By carefully and judiciously employing AI approaches, it is possible to improve anesthesia-related tasks and patient care. Additionally, given the importance of interpretability in medical decision-making, using interpretable AI techniques is strongly recommended for future studies. These methods allow physicians to analyze and understand the results, leading to more confident and informed decisions. Overall, the integration of AI in anesthesia holds promise for optimizing patient outcomes and the overall efficiency of anesthesia management. As technology advances, researchers and practitioners should continue exploring innovative AI applications to further revolutionize the field of anesthesiology.