1. Introduction
In late 2019, a novel viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged from China, spread worldwide, and became a global concern (1). It has been demonstrated that coronavirus disease-19 (COVID-19) may present with mild and nonspecific symptoms in the pediatric population in contrast to adults (1). While the most common clinical manifestations of SARS-CoV-2 infection in pediatric and adult populations are related to the respiratory system, some clinical manifestations, including neurological deficits, are not commonly reported among children (1, 2). Currently, more than 1000 publications are addressing neurological manifestations of SARS-CoV-2 infection. However, some neurological manifestations, including transverse myelitis, are extremely rare in children (3). Transverse myelitis (TM) is a clinical syndrome in which one section of the spinal cord has sustained a bilateral neurological deficit secondary to an underlying immunological process. Transverse myelitis can result in various autonomic dysfunction, weakness, and sensory changes. We report a case of acute transverse myelitis in a pediatric COVID-19 patient, which to our knowledge, is one of a few cases from Iran and the world.
2. Case Representation
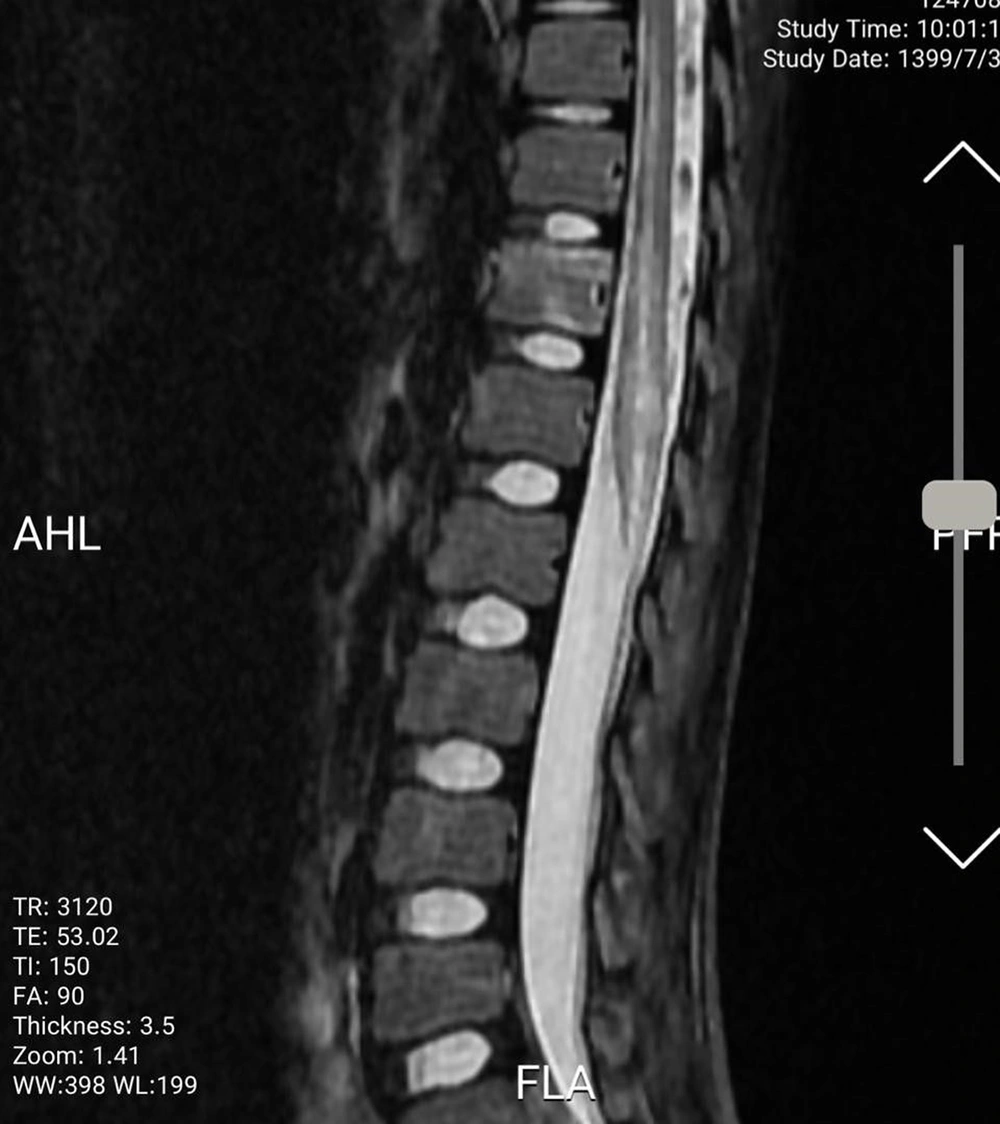
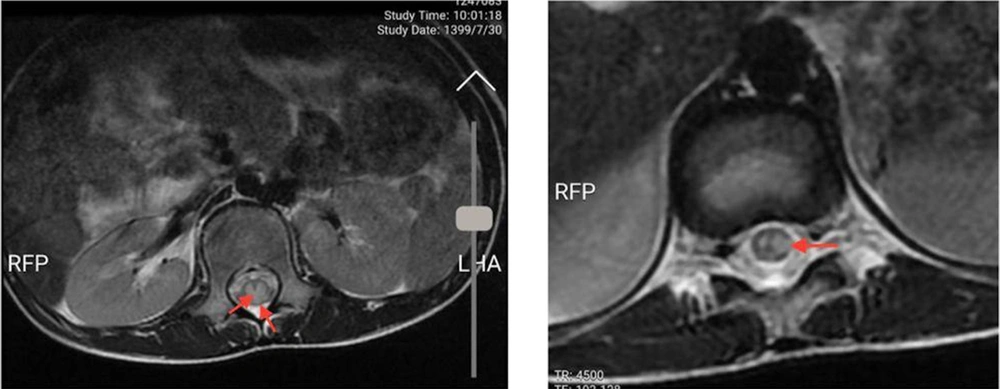
An 11-year-old previously healthy female patient was brought to the emergency department due to limb paralysis. The patient’s symptoms began one week prior to admission with fever. Four days prior to the admission, the patient developed acute paraplegia. One day after the development of paraplegia, the patient developed urinary retention and constipation. There was no history of respiratory symptoms since the onset of the fever. Moreover, none of her family members had similar clinical manifestations and was not tested for COVID-19. At the time of admission, the patient was febrile (39°C axillary), conscious and the vital signs were stable (systolic/diastolic blood pressure: 110/70 mmHg; respiratory rate: 25 breaths/minute; heart rate: 90 beats/minute). The neurological examination revealed intact cranial nerves, reduced lower limb power (I/V), and reduced deep tendon reflexes. The flexor plantar reflex was normal, and there was sensory loss below the level of the umbilicus (T10 sensory level). Other physical examinations were unremarkable. No other organ involvement was detected. While complete blood count and liver function tests were normal, elevated C-reactive protein (96 mg/L (normal range: < 10 mg/L)) and erythrocyte sedimentation rate (62 mm/h (normal range: < 22 mm/h) were noted. All other blood chemistry tests were normal, including lactate dehydrogenase, creatinine phosphokinase, and coagulation study. The CSF analysis and oligoclonal band screening were unremarkable, which ruled out multiple sclerosis. Blood and CSF cultures did not show any bacterial growth. Brain computed tomography scan and magnetic resonance imaging (MRI) were unremarkable. The spine MRI revealed continual abnormal signals in the lower spinal cord region (Figures 1 and 2). The reverse transcript polymerase chain reaction (PCR) of SARS-CoV-2 from nasopharyngeal samples was positive. Virology investigation results for cytomegalovirus, human herpes virus, herpes simplex virus, varicella-zoster virus, Epstein Barr virus, rubella, measles, mumps, parvovirus B-19, influenza A and B, adenovirus, parainfluenza viruses, and rhinoviruses were negative.
The patient received methylprednisolone (500 mg) for three days and two doses (1 g/kg for each dose) of intravenous immunoglobulin (IVIG). Additionally, the patient received atazanavir/ritonavir (ATV/r) 300 mg/100 mg once daily. On day seven of admission, the neurologic examination revealed normal sensory findings. While the right lower limb had normal deep tendon reflex and muscle force, the left lower limb still had reduced deep tendon reflex, and the patient could not move her left leg.
3. Discussion
The present report demonstrated successful management of acute transverse myelitis in a pediatric patient with COVID-19. Pediatric COVID-19 accounts for less than 5% of COVID-19 cases, and most pediatric patients become mildly symptomatic or remain asymptomatic (1). Neurological manifestations, including headache, anosmia, and dizziness, are common extrapulmonary manifestations of COVID-19. Although the exact mechanism behind the neurological manifestations in COVID-19 patients is still unclear, neuro-inflammatory injury induced by cytokine storm is considered a possible cause of neurological deficits (4). Various inflammatory cytokines, including interleukin-6 (IL-6), may aggravate neuronal damage and induce neurological symptoms (4). Acute transverse myelitis is a subgroup of non-compressive myelopathies with variable outcomes (5). Although transverse myelitis mainly occurs in adults, approximately a quarter of cases are reported in the pediatric population (6, 7). Among pediatric patients, those younger than three years or those between 5 and 17 years are most likely to be affected by transverse myelitis (8). While pediatric transverse myelitis is idiopathic in some individuals, RNA and DNA viruses are considered the leading causes of transverse myelitis (9). Among the viral diseases, varicella-zoster and enteroviruses are the most common viruses causing transverse myelitis (9). Acute transverse myelitis develops from hours to several days post-infection (8). The most common manifestation of transverse myelitis is the development of bilateral weakness and sensory disturbances below the lesion’s level (8).
In our patient, acute presentation of sensory loss below the level of the umbilicus and paraplegia were the most evident findings indicating the neurological deficit. Due to positive nasopharyngeal COVID-19 PCR and negative viral and bacterial studies of blood and CSF, in addition to no other signs and symptoms suggesting other neurological, autoimmune, or infective causes, we believe COVID-19 is the most likely cause of LETM in our patient.
Globally, a small number of transverse myelitis cases secondary to COVID-19 infection in pediatric patients have been reported.
Khera et al. reported a case of longitudinal extensive transverse myelitis (LETM) and concomitant GBS in an 11-year-old COVID-19 patient (10). She did not have any viral prodrome and developed urinary and bowel incontinence. This was in contrast to our patient, who developed a fever and bladder and bowel retention. The patient developed a progressive weakness for two days, leading to acute flaccid paralysis and the need for respiratory support and intensive care unit admission. Imaging findings showed an acute lesion in the brain, unlike our patient, and enhancement of cauda equina lesion in keeping with GBS and LETM. The patient did not respond adequately to intravenous pulse methylprednisolone and intravenous immunoglobulin therapy; therefore, she was treated with plasma exchange therapy, leading to nearly full recovery (10).
Guler et al. reported the case of a 14-year-old patient who presented with right-sided hemiplegia, unlike our patient, and had otherwise normal physical and neurological examination findings and no viral prodrome (11). The patient had a positive nasopharyngeal COVID-19 PCR. Coronavirus disease 2019 CSF PCR and IgM/IgG were not performed. The patient was initiated on IVIG, to which she did not respond; therefore, she was initiated on pulse methylprednisolone therapy which improved her symptoms (11).
Poyrazoglu et al. reported a 10-year-old patient who developed acute demyelinating encephalomyelitis (ADEM) and transverse myelitis (12). Patients’ symptoms began with fever and headache and progressed to weakness and inability to walk. The patient had a positive COVID-19 PCR for CSF and a nasopharyngeal swab. He received empirical antibiotics, aciclovir, plasmapheresis, IVIG, and methylprednisolone (12).
Nejad Biglari et al. reported an 11-year-old patient who presented with lower limb paraplegia and urinary and bowel retention (13). She was initially treated with methylprednisolone pulse and IVIG treatment. Due to a lack of response, she was commenced on seven cycles of plasma exchange which failed to improve her paraplegia. Unlike our patient, she had epigastric pain and fever before developing neurological symptoms. CSF PCR and viral antibody testing were not performed at the time of admission; however, the patient had a positive nasopharyngeal COVID-19 PCR. Like our patient, her brain imaging showed no abnormalities; however, her spinal MRI indicated transverse myelitis (13).
Naseri et al. reported the case of a 4-year-old girl who presented with upper limb paresis, lowers limb paraplegia, and absent deep tendon reflexes (14). Before admission, she had suffered from symptoms including weakness, lethargy, constipation, severe back pain, and shoulder pain. Examinations revealed bilateral sixth nerve palsy, absent gag reflex, and tongue biting-like movements (14).
Brisca et al. reported a case of transverse myelitis secondary to COVID-19 infection in a 7-month-old girl (15). She had an acute inability to sit up following respiratory symptoms a week prior. CSF analysis was negative. Nasopharyngeal COVID-19 PCR was negative, and her MRI indicated LETM. She also had concomitant myopericarditis. She had a satisfactory recovery after steroid and IVIG therapy (15).
Jagadish et al. reported the case of a 5-year-old male who presented with an altered mental state and vomiting (16). He was intubated as he was in respiratory distress. He tested positive for nasopharyngeal COVID-19 PCR, and his chest X-ray revealed right lower lobe consolidation. Dexamethasone was initiated, and sedation was weaned off to extubate the patient; however, it was discovered that the patient could not move his extremities and had a minimal cough and gag reflex. Spinal MRI revealed LETM; however, his CSF analysis was normal. He was prescribed solu-medrol and intravenous remdesiver and received plasmapheresis. Despite minor improvement, it was deemed necessary for him to undergo gastrostomy and tracheostomy (16).
Grasso et al. reported the case of a 12-year-old boy with an asymptomatic COVID-19 infection (17). The patient complained of nuchal pain followed by hypoesthesia on the right hemisoma that persisted for twelve hours. His brain MRI was normal, and his spinal MRI indicated longitudinal extensive transverse myelitis. His CSF and blood virology, and microbiology were negative. His CSF COVID-19 genome sequencing and the oligoclonal band were negative. The patient received aciclovir, azithromycin, and high-dose intravenous methylprednisolone and fully recovered in 11 days (17).
Kaur et al.’s study reported a 3-year-old COVID-19 patient presenting with quadriparesis and respiratory failure (18). Treatment was initiated with intravenous methylprednisolone and immunoglobulin and escalated to plasma exchange and rituximab as the patient developed more prominent oedema (18).
Appropriate and prompt treatment is an important issue regardless of the underlying aetiology etiology of acute transverse myelitis. While the combination of IVIG and corticosteroids was unsuccessful in the Kaur et al. patient, we decided to add antiviral treatment instead of performing therapeutic plasma exchange and demonstrated that our patient responded well to the therapy (18). It has been demonstrated that COVID-19 pediatric patients with multisystem inflammation syndrome respond well to IVIG and steroid therapies without any neurologic sequelae; therefore, we decided to initiate the treatment with IVIG and corticosteroids.
3.1. Conclusions
In conclusion, acute transverse myelitis is a rare neurological finding in the pediatric population. Our report emphasizes that during the COVID-19 outbreak, the physician should consider SARS-CoV-2 as a possible cause of acute transverse myelitis in children and may successfully treat the condition by considering corticosteroids, IVIG, and antiviral therapy.