1. Introduction
Nephrotic syndrome (NS) is a condition characterized by swelling, proteinuria, and hypoalbuminemia with or without hyperlipidemia. The most common cause of NS is idiopathic. The incidence of NS is 1.15 - 16.9 in every 100,000 children. The highest incidence is in Asia compared to Europe (1). One of the NS complications is arterial thromboembolism (ATE) due to a hypercoagulable state, occurring in 1.5% of cases annually (2). Nephrotic syndrome causes the loss of low molecular weight proteins, including plasminogen and antithrombin III, that could lead to impaired regulation of coagulation. Hypoalbuminemia in NS induces increased hepatic synthesis of clotting factors (3). Idiopathic NS can be classified according to the response to steroid therapy, histopathology, or a genetic mutation. The main treatment for NS is corticosteroids (4).
Arterial thromboembolism that causes stroke can also happen in a child with NS. The incidence of ATE is 1.5% annually, with complications of myocardial infarction, peripheral artery disease, and ischemic stroke. The risk factors of ATE in NS patients are the severity of hypoalbuminemia and proteinuria, infection, hypovolemia/hemoconcentration, use of corticosteroid and diuretics, and loss of molecular weight molecules and antithrombotic. Several cases of stroke in NS were diagnosed mostly in a patient with minimal changes disease (18%), membranoproliferative glomerulonephritis (14%), focal segmental glomerulonephritis (5%), and IgA nephropathy (5%). Some studies have concluded that the risk of thrombosis is highest shortly after the diagnosis of NS (3).
In NS, prophylactic anticoagulation and thrombosis treatment are not clearly established due to the lack of a large randomized trial. Guideline-related anticoagulant dosing for NS depends on the patient’s condition. In this case, we administered anticoagulation as a prophylactic agent during transient high-risk events or as treatment in thromboembolic events (5).
We encountered a 9-year-old boy with NS and infarct stroke, which was treated with corticosteroid and low molecular weight of heparin with clinical improvement. Informed consent was obtained from the patient’s guardian to publish the case details and accompanying images.
2. Case Presentation
A 9-year-old boy complained of decreased consciousness 1 month before admission, followed by seizures with upward eyeballs accompanied by tonic-clonic movement. The seizure occurred 3 times a day with a duration of 10 - 15 minutes each time. The patient complained of the first seizure 1 month before admission. During the seizure, the eyeballs rolled upward (accompanied by tonic-clonic movement), followed by swelling of the body a few weeks before admission. The patient also complained of tea-colored urine. It showed hematuria that might be glomerular. The seizures occurred 3 times with a duration of 10 minutes. After that, his face became asymmetric, and he could not speak fluently. The patient was referred to a local hospital due to a seizure. Vital signs were checked and revealed high blood pressure, 180/100 mmHg. The patient received an antihypertensive agent. Swelling of the body occurred 8 months before admission. The patient was diagnosed with NS and was given prednisone and albumin orally. The patient was referred to our hospital for further treatment. The clinical history of the patient is described in Figure 1.
At the emergency room, he experienced decreased consciousness with a Glasgow Coma Scale (GCS) of E4M5V2 and a blood pressure of 120/100 mmHg. The pulse rate, respiratory rate, and body temperature were normal. On general examination, there was periorbital swelling, fluid wave in the abdomen, and bilateral trunk swelling. The neurological examination showed an upper motor neuron lesion with increased physiological reflexes, positive Babinski sign, right hemiparesis, and clonus.
Laboratory examination showed an Hb level of 10.1 g/dL, leucocyte of 11.760/mm3, hematocrit of 28.5%, platelet of 713,000/mm3, serum albumin level of 0.4 g/dL, serum potassium level of 2.5 mEq/L, serum urea level of 28 mg/dL, and serum creatinine level of 0.96 mg/dL (glomerular filtration rate of 73 mL/minutes/BSA). On urinalysis findings, there was +4 protein, with a 24-hour urinary protein quantitative result of 4866 mg/dL. During hospitalization, thromboelastography (TEG) was examined and revealed hypercoagulation; also, there was an increase in D-dimer and fibrinogen levels. We performed the International Normalized Ratio (INR) examination every 24 hours.
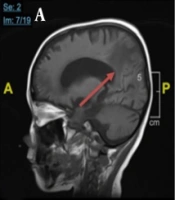
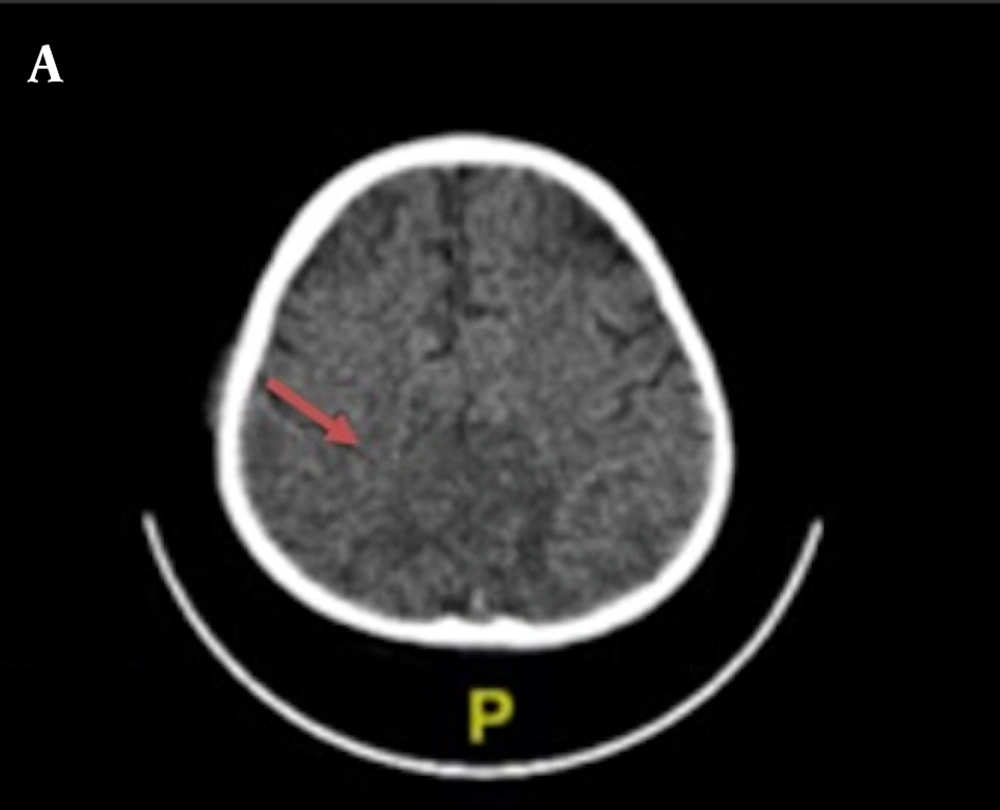
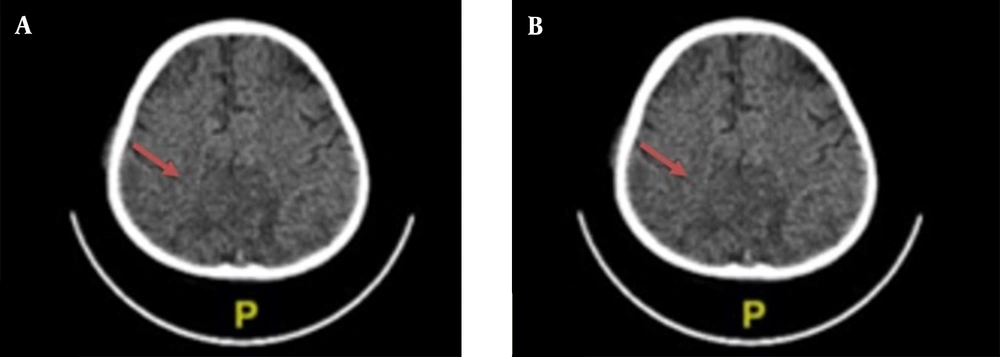
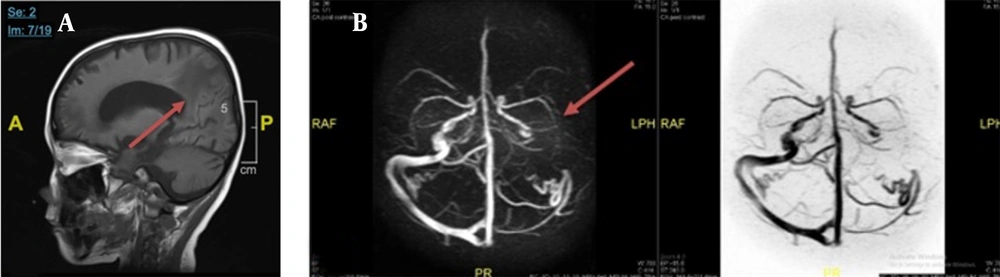
Chest X-ray examination revealed no bronchopneumonia or cardiomegaly. It showed mild pleural effusion, with normal heart size, supporting underfilled pathophysiology as in idiopathic NS. A head computed tomography (CT) scan showed a hypodense lesion predominant on the left basal ganglia, subcortical right parietal lobe, and bilateral parietal due to suggestive posterior reversible encephalopathy syndrome (PRES) differentiated with cerebral infarction, left temporal lobe, left external capsule, and cephalhematoma at the right temporoparietalis region (Figure 2). Head CT scan showed suspect cerebral infarction on the cortical temporal lobe and left external capsule, cephalhematoma on the right temporo-parieto-occipitalis, mastoiditis dextra, and cerebral atrophy with ventriculomegaly ex vacuo (Figure 3). A head magnetic resonance imaging (MRI) scan showed a chronic infarction in cortical-subcortical fronto-temporo-parieto-occipital lobes, suggestive left sinus transversus thrombus, ventriculomegaly, and right concha hypertrophy (Figure 4).
During the treatment, the patient received albumin transfusion, methylprednisolone (16 mg; 2 tablets each morning in alternate dose), captopril as an antiproteinuric agent, calcium carbonate (500 mg) once a day, calcitriol (0.5 μg) once a day, cyclophosphamide, and low molecular weight heparin (LMWH) (20 mg) subcutaneously once a day with coagulation factor examination every 24 hours. Low molecular weight heparin was given as a prophylactic agent of thrombosis in NS until an INR target of more than 1.5 and/or a normal level of albumin. The patient was discharged from the hospital and went to the outpatient clinic with clinical improvements, including increased motor strength and improved consciousness.
3. Discussion
Nephrotic syndrome is a condition characterized by swelling, proteinuria, and hypoalbuminemia that can be accompanied by hyperlipidemia (4). Nephrotic syndrome is classified as a minimal or non-minimal lesion. Nephrotic syndrome with non-minimal lesions is the most common in ages under 1 year old or over 7 years old. This type of NS is associated with complications such as acute circulatory collapse, thrombosis, and lung or cardiac embolism. It is also associated with chronic complications due to immunocompromised and infectious conditions (4). Also, this kind of NS is closely associated with the high expression of pro-inflammatory mediators, which might increase the risk of cardiovascular remodeling and abrupt coagulation (6).
The risk factors of hypercoagulation in NS are genetics, thromboembolic history, albumin level of less than 0.2 mg/dL, using corticosteroids, and steroid-resistant NS. In this case, hypercoagulation happened due to low albumin levels. It might be due to the excessive loss and inadequate intake of the patient. Nephrotic syndrome causes the loss of low molecular weight proteins, including plasminogen and antithrombin III, which could lead to impaired regulation of coagulation. Hypoalbuminemia in NS induces increased hepatic synthesis of clotting factors. The hypercoagulable state of the patient with NS results from platelet activation and aggregation changes. It is also caused by an imbalance of procoagulant/prothrombotic and anticoagulant/antithrombotic factors, leading to thrombosis in the deep vein arteries. Arterial thrombosis occurs mostly in children. Small artery occlusion is the leading cause of ischemic stroke in NS patients (7-9). The risk factors of ATE in the patient were infection, severe volume depletion, and severe thrombocytosis.
Our NS patient had an arterial ischemic stroke with an albumin level of less than 3 g/dL and cholesterol serum of more than 250 mg/dL. The patient also showed heavy proteinuria (8). Cholesterol is one of the causes of cerebral infarction (10). In this case, we did not give anti-lipid because the clinical signs were significantly improved only by decreasing the blood pressure and managing the platelet aggregation with LMWH. We consider that hyperlipidemia in NS is mostly not malignant because of the molecular adhesion in NS, which is usually different from other causes. Molecular adhesion (such as vascular cell adhesion molecule (vCAM)) is responsible for atherosclerotic formation (11). Nevertheless, we educated the parents to give a good nutrition intake to the patient related to his hyperlipidemia.
In NS, prophylactic anticoagulation and thrombosis treatment are not clearly established due to the lack of a large randomized trial. Guideline-related anticoagulant dosing for NS depends on the patient’s condition. In this case, we administered anticoagulation as a prophylactic agent during transient high-risk events or as treatment in thromboembolic events. Based on Kidney Disease: Improving Global Outcomes (KDIGO) 2020, full-dose prophylactic anticoagulation should be considered if there are serum albumin < 2.0 - 2.5 g/dL, proteinuria > 10 g/dL, and prolonged immobilization (5). The decision to give anticoagulants to the patients is based on several factors, notably for those with a high risk of thromboembolic events (immobility) like in our patient. No levels of proteinuria and albuminemia have been studied to start anticoagulation. Conventional anticoagulation (such as heparin or warfarin) remains the standard therapy (9). The patient took medicine regularly as suggested by the doctor. Parents see the clinical improvement and still take their children to an outpatient clinic on schedule. The participant gave consent to participate and publish the data.
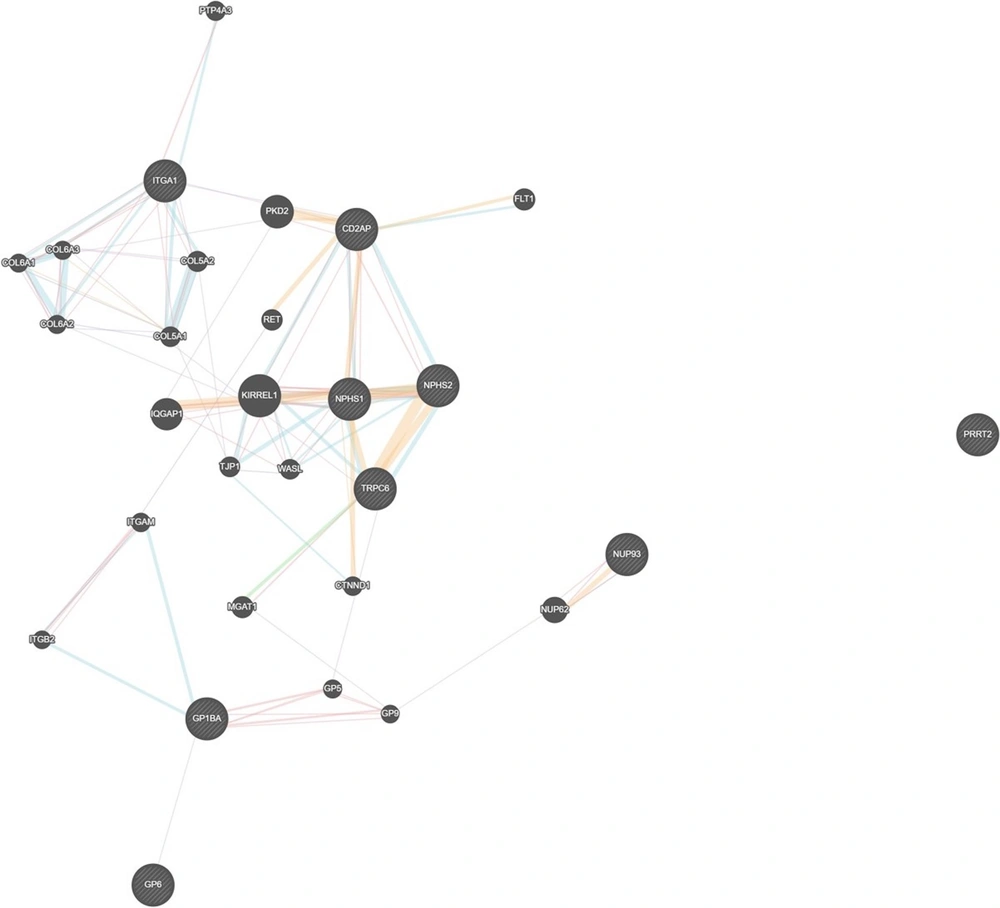
In recent years, the molecular basis of the NS has placed the podocyte as the target cell related to the development of proteinuria (12). Based on in silico analysis, the proteins responsible for the podocyte integrity and having a connection with platelet aggregation are integrin subunit beta (ITGB)1 and transducin repeat-containing protein (TrPC)6. Also, higher ITGB1 expression correlates with a good prognosis (13). ITGB1 has the same pathway as glycoprotein 9 (GP9), which is responsible for platelet aggregation disorders. Also, ITGB1 has the same pathway as glycoprotein 1B protein sub-alpha (G1BA). However, TRPC6 is almost always co-expressed with GP9 (Figure 5). Figure 5 shows gene interaction. It also describes in silico analysis that relates the proteins responsible for podocyte integrity and platelet aggregation (14). We sought to investigate the propensity of NS to ATE by linking proteins involved in podocyte integrity and coagulopathy. Our search process was done by in silico analysis based on the gene that codes for those proteins.
3.1. Limitations and Recommendations
The early diagnosis and proper treatment of thromboembolic events can prevent complications and readmission and decrease the mortality rate. Furthermore, overuse or misuse of various anticoagulants in the management of this issue could lead to increased complications. Accordingly, guidelines can help with the proper management of children with such diseases, as seen in the precise antibiotic use in various infectious diseases (15).
3.2. Conclusions
Thrombocytosis in NS children tends to hypercoagulability entity. Hence, the clinician should be aware of thromboembolism that can affect essential organs, notably the heart, lungs, and brain. This report reflects that we should not underestimate the high platelet level. We propose aggressively performing some TEG examinations of all NS children with high platelet levels. We also recommend the use prophylactic anticoagulation with monitoring of bleeding risk during therapy.