1. Background
Cystic Fibrosis (CF) is the most common fatal genetic disorder in white-race communities with a prevalence of 1:2500. Mutation in CFTR gene is its main cause (1-4), yet the reason of this genetic anomaly is unknown (3). Clinical progression of CF is highly variable. While malabsorption is the initial sign, other frequent manifestations are fatty diarrhea, recurrent respiratory infections, and failure to thrive (FTT) (5, 6). The main reason of mortality and morbidity in CF patients is pulmonary involvement (7-9). In various studies, it has been shown that quantitative evaluation of parenchymal changes in lung HRCT of CF patients measured by the 2006-revised Brody scoring is compatible with the clinical status (1-6). Patients with CF are mostly children, unable to perform complicated pulmonary tests. On the other hand, screening tests are not performed in many countries, such as Iran, resulting in late diagnosis in older ages with more diffuse parenchymal involvement and longer hospitalization. Consequently, the quantitative measurement of parenchymal changes in lung high-resolution computed tomography (HRCT) seems to be useful for early and accurate evaluation of the patients’ clinical status.
2. Objectives
Since no study has been performed in this regard in Iran, the aim of the present research was to determine the severity and prevalence of pulmonary changes in late diagnosed patients with CF by means of HRCT Brody scoring system.
3. Patients and Methods
In this cross-sectional study, 23 CF patients (diagnosis of whom certified by sweat test) aged 5 - 18 years (Mean: 13.42 ± 3.6) were selected from the patients hospitalized in Masih Daneshvari Hospital from 2006 to 2009. The study was approved by Ethics Committee of Masih Daneshvari Hospital. Informed consent was obtained from all patients participating in the study. HRCT was performed during a deep inspiration from the apex to the base of lungs and also in the expiratory phase if the patient cooperated. The slice thickness and interval were 1 mm and 20 mm, respectively. The CT scans were obtained with a spiral CT unit (Siemens SOMATOM Emotion Series, Siemens Erlangen, Germany).
An attending expert radiologist in pulmonary diseases and two residents in radiology scored the HRCT based on the Brody scoring system with consensus. Five variables in central and peripheral parts of six lobes of lungs were scored, including bronchiectasis and peribronchial wall thickening (PBWT), mucous plugging (MP), parenchymal changes (ground glass opacity (GG) and consolidation (CON)), air trapping (AT) and the total score was calculated. The 2006-revised Brody scoring system is a tool to score the five variables found by HRCT. The least possible total score in the scoring system is zero and the highest is 243 points. Data were analyzed with SPSS software version 17 (SPSS Inc., Chicago, the USA). P value < 0.05 was considered statistically significant.
The HRCT scoring system (Alen S. Brody, March 2006):
Bronchiectasis = (extent in central lung + extent in peripheral lung) × average size multiplier (range: 0 - 12).
Mucous plugging = extent in central lung + extent in peripheral lung (range: 0 - 6).
Peribronchial wall thickening = (extent in central lung + extent in peripheral lung) × severity (range: 0 - 9).
Parenchymal score = extent of dense opacity + extent of ground glass opacity + extent of cysts or bulla (range: 0 - 9).
Air trapping score = extent of air trapping × Appearance of air trapping (range: 0 - 4.5).
4. Results
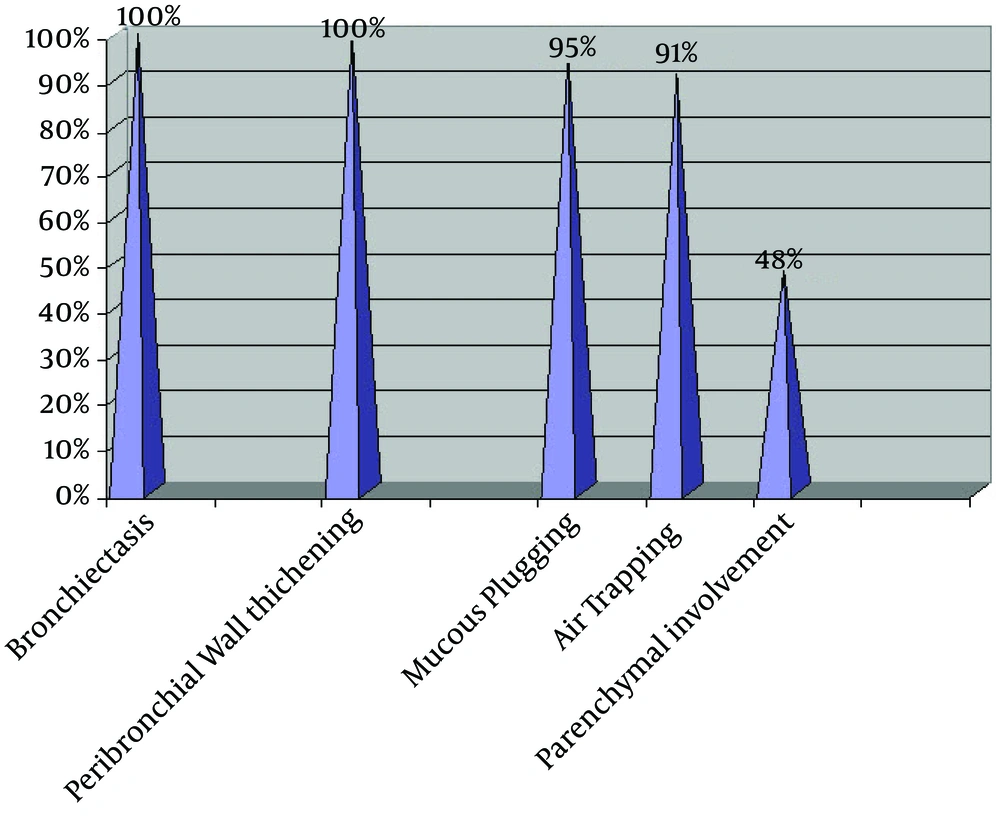
Of the total 23 patients, nine (39.1%) were female and 14 (60.9%) male. The overall CT score for all patients was 57.6 ± 24.2. The prevalence of HRCT findings showed bronchiectasis and PBWT in all patients (100%), MP in 95.7%, AT in 91.3%, and parenchymal involvement in 47.8% of them (Figure 1).
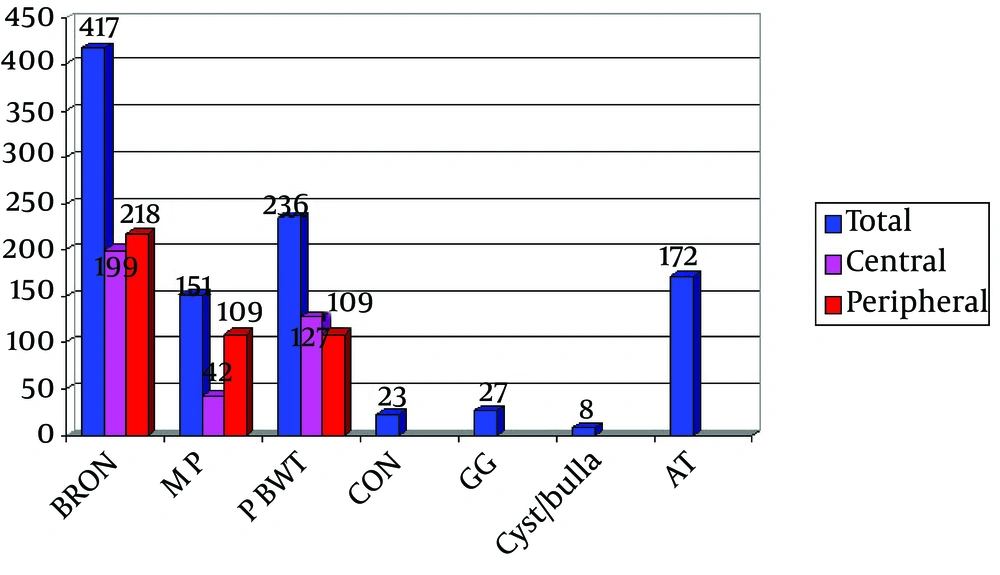
From total of 1034 points, 417 score was related to bronchiectasis and this score is significantly higher than the scores of other parameters (Parenchymal changes: 58, MP: 151, AT: 172, PBWT: 236) (Table 1 and Figures 2 and 3).
aAbbreviations: AT, air trapping; LLL, left lower lobe; LUL, left upper lobe; MP, mucous plugging; PBWT, bronchiectasis and peribronchial wall thickening; RLL, right lower lobe, RML, right middle lobe; RUL, right upper lobe.
The score for parenchymal changes was also significantly lower compared to other parameters. From a total score of 1034, contribution of the lobar involvement had the following order: right upper lobe (RUL): 206 > left lower lobe (LLL): 188.5 > right lower lobe (RLL): 186.5 > left upper lobe (LUL): 177 > Lingula, right middle lobe (RML): 138, which did not show significant difference. The highest score in all lobes was related to bronchiectasis and the lowest to parenchymal changes. Comparing the parameters in the central and peripheral areas, only MP in the peripheral zone was significantly higher than in the centrum (Figure 4). Other changes were not significantly different in central and peripheral areas. Bronchiectasis, MP, PBWT, and parenchymal changes did not show significant difference in lobar distribution while AT was significantly less important in the middle lobes (Figures 5 and 6). The total scores of the parameters in different pulmonary lobes for patients with CF are summarized in Table 1.
5. Discussion
CF is known as the most common fatal genetic disease among the white population (10, 11).
Many developing countries do not offer screening and genetic testing, and the underlying pulmonary disease in children is diagnosed after recurrent pneumonitis at older ages in special referral centers. This delayed diagnosis leads to parenchymal involvement and especially irreversible changes such as bronchiectasis. Pulmonary lesions are more frequently seen in central and upper lung regions. In this study with 23 patients, five variables were evaluated and significant differences in the lobar or degree of zonal involvement (central or peripheral) were not found, while involvement of the lower lobes was observed more often compared with the left upper lobe. Although MP score was higher in peripheral areas, due to advances in disease treatment, the score for bronchiectasis was significantly higher whereas the score for PBWT was not significantly more important than other variables. Additionally, all study patients had bronchiectasis and PBWT, which were more prevalent compared to the related studies.
In a study by Dorlochter et al. which enrolled 17 CF patients with a mean age of 5 years, 13 individuals had bronchiectasis and 10 cases had MP (12). Yet, in a study by Bhalla et al. including 14 patients with CF between 4 - 10 years old, similar results with our study were obtained regarding the prevalence and severity of bronchiectasis and PBWT in all lobes (13). In another study by Dakin et al. the prevalence of bronchiectasis, PBWT, and MP in 19 of the total patients with CF with an average age of 9 years was less than that found in our study. However, the lobar involvement was similar to our data (RUL > LLL > RLL > LUL > Lingula > RML) (14).
In the present study, the most common cause for visit and hospitalization of patients with CF was pneumonia and increased infection in thickened secretions. In HRCT, it presented as GG opacities and consolidation. This pathophysiology suggests that the scores of related parameters should be higher in theory, but in reality they were significantly lower than other parameters. It appears that in the initial phase of the disease, the infectious processes cause parenchymal involvement similar to any pneumonia GG opacity and alveolar consolidation; but, as the disease progresses and both lungs have parenchymal destruction and formation of bronchiectasis takes place, secondary infections present less in the form of typical GG and consolidation. Additionally, lack of such presentation in the HRCT of adults does not rule out the secondary infection, but its presence can be helpful. In regards to the AT parameter, significantly lower values were found in the middle lobes (Lingula and RML). However, because of the lack of ideal cooperation by children in obtaining HRCT in expiration, our scoring for this parameter by the Brody system was not reliable.
It seems that in patients with advanced CF lacking the appropriate medical care, severity, prevalence, and appearance of lung lesions do not follow a definite specific pattern. However, a similar study in older patients with a larger sample size for comparison of the results is suggested.