1. Background
John Langdon Down first defined Down syndrome (DS) as “a distinct form of mental disability” in 1862. He used the term “Mongoloid” because of his perception that children with DS shared facial similarities (epicanthal folds) with those of Blumenbach's Mongolian race.
With the discovery of karyotype techniques in the 1950s, Jerome Lejeune discovered that DS results from an extra chromosome. In 1959, the extra chromosome was labeled as the 21st, and DS began to also be referred to as “trisomy 21.”
In 1961, eighteen geneticists wrote to the editor of The Lancet suggesting that the term “Mongolian idiocy” had “misleading connotations” and that it had become “an embarrassing term” that should be changed. Finally, The Lancet supported the use of the term DS (1).
DS is the most common chromosomal abnormality in newborns. The specific abnormalities that are seen in DS children include varying dysmorphic features and congenital malformations. Some individuals with DS are profoundly impacted, whereas others are able to live independently in some respects.
More than 80 clinical features are seen in DS, including cognitive impairments, muscle hypotonia, short stature, facial dysmorphisms, congenital heart disease, and several other anomalies. Leukemia and Hirschsprung disease occur more frequently in patients with DS than in the general population (2-4).
Although there is some evidence that the prevalence of DS varies among racial and ethnic groups, the general prevalence of DS in live births is approximately 1 in 750 (5-7). The occurrence of trisomy 21 as well as other autosomal trisomies increases with advanced maternal age (≥ 35 years). Younger mothers represent half of all mothers with babies with DS because of their higher overall birthing rate, but it is believed that younger women have a lower risk (8).
Many epidemiological studies about the prevalence, causes, and clinical significance of DS have been conducted over the last 100 years, and it has been determined that DS occurs in all ethnic groups and among all economic classes. At the maternal ages of 20 to 24, the probability of having an infant with DS is one in 1562; at ages 35 to 39, the probability is one in 214, and above age 45, the probability is one in 19. Recent data also suggest that paternal age, especially beyond 42, also increases the risk of DS (9, 10); however, the impact of paternal age is very low according to current knowledge (11).
In one study, the mean age of Iranian mothers with DS children was found to be six years less than the mean age of mothers with DS children in Western countries (12).
Many studies have assisted us in understanding the epidemiology of DS and its geographic variations (13-17).
Kallen et al. studied major malformations in 5581 infants with DS from three registers of congenital malformations. They compared the prevalence between DS and non-DS infants. In DS infants, they identified increased risk for annular pancreas, cataracts, duodenal atresia, megacolon, small choanal atresia, esophageal, anal and small bowel atresia, pre-axial polydactyly, and omphalocele. Increased risk in DS infants was also identified for other abnormalities, such as cleft palate, cleft lip/palate, and limb deficiencies. No increased risk was seen for neural tube defects, hydrocephaly, microtia, renal or severe dysgenesis, hypospadias, or for any forms of polydactyly other than pre-axial. Cardiac defects were registered in 26% of all DS cases (which varied between different registeries programs), and 28% of these cardiac defects were unspecified (18).
The DS phenotype is complex and varies among individuals, ethnicities, and countries; several studies on DS have been conducted in developed countries (19-21). However, our knowledge about the frequency of DS-related complications and their relations to co-morbidity and ageing is limited, especially in developing countries.
2. Objectives
In this study, we analyzed the clinical characteristics of DS patients in Mashhad (a large city northeast of Iran with a population of approximately 2749374) who were referred to the pediatric cardiology department of an educational hospital.
3. Patients and Methods
In this cross-sectional study, over 11 years (from September 2001 to September 2012), we studied 100 children with DS and heart defects who were referred to the pediatric cardiology department of an educational referral hospital affiliated with the Mashhad University of Medical Sciences. The inclusion criterion was DS children who had been referred to the pediatric cardiology department during the 2001-2012 period. Patients who were not referred for follow-up and treatment and patients who died before the start of treatment were excluded.
All data were collected according to a checklist designed by the researchers, which included demographic and clinical information, genetic characteristic, cardiac and non-cardiac co-existing diseases, and the parental variables of the children. These data were obtained from patient files or by taking a history from the parents of the patients and through direct follow-up with the patients by a pediatric resident. All analyses were conducted using SPSS version 11.5. In this study, statistical tables and charts were used to describe subject data. quantitative variables such as birth weight and age of diagnosis were analyzed by an independent t-test, and the chi-square test was used to compare qualitative variables. P < 0.05 as considered statistically significant.
This study was approved by the ethical committee of the Mashhad University of Medical Sciences.
4. Results
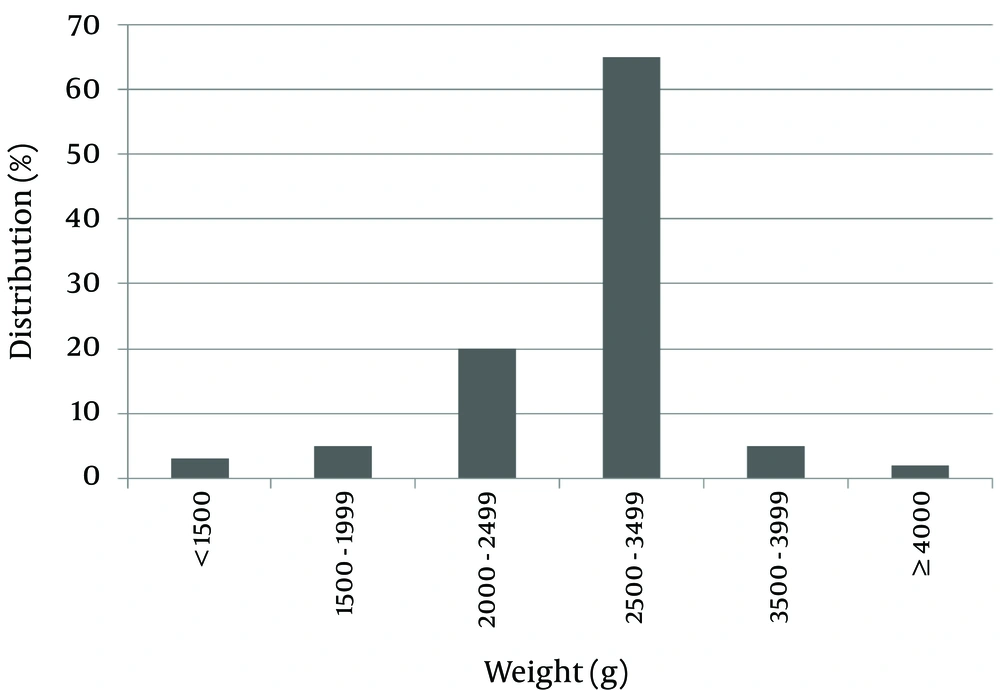
One hundred patients with DS were evaluated; 52 of them were female (52%) and 48 were male (48%). The average birth weight of the subjects was 2,744.80 ± 522.83 grams. Maximum and minimum birth weights were 1200 and 4900 grams. For 65% of subjects, birth weight was in the range of 2500 - 3500 grams.
Of the patients studied, 28% were small for gestational age (SGA), and 7% had a birth weight greater than 3500 grams. Only two patients (2%) were large for gestational age (LGA) (Figure 1).
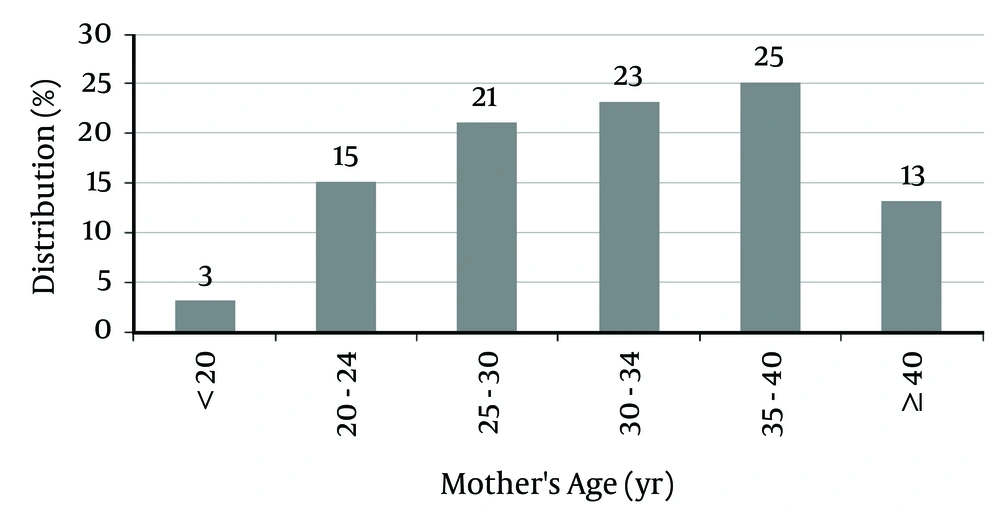
The mean mothers’ age for patients was 32.04 ± 6.03 years (between 18 and 42 years). Of the mothers, 38% were older than 35 years, and 62% were younger than 35 years (Figure 2).
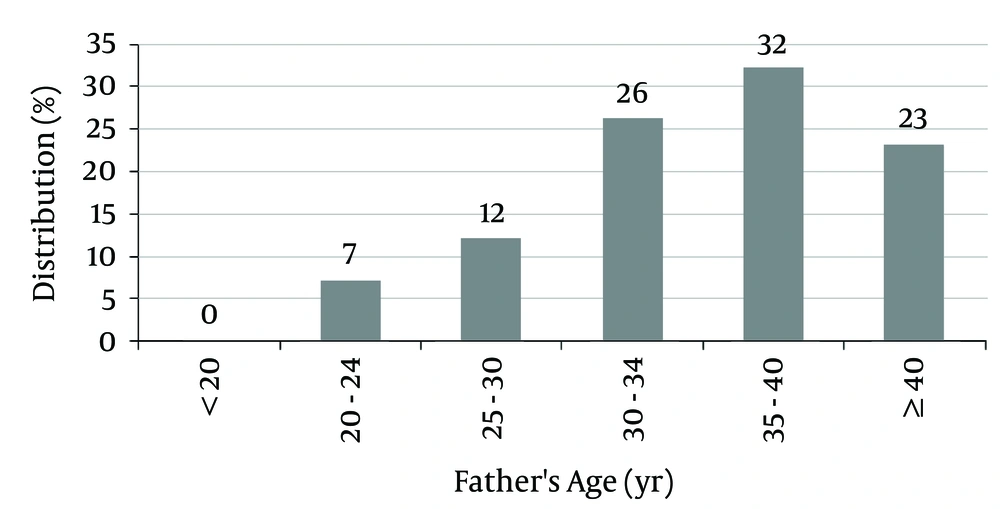
The mean fathers’ age of patients was 35.55 ± 6.02 years (between 21 and 48 years).
Of the fathers, 55% were older than 35 years, and 23% were older than 40 years at the birth time of the subjects (Figure 3).
Genetic assessment was proposed for every patient, but some parents refused (The parent of 39 patients).
Chromosomal analysis was performed for 61 patients, and the chromosomal findings in 60 (98.40%) indicated free trisomy. One patient had translocation (1.60%), and no patients had a mosaic pattern of chromosomal abnormality.
In 33 cases, the parents of the patients were consanguineous (third degree, distant relatives). In three cases, there was a familial history of Down syndrome; the translocation case was among these.
All patients had cardiac disorders (Table 1), and non-cardiac disorder was recorded in 37 patients (37%). The most common non-cardiac disorder was hypothyroidism, and the second most common was gastrointestinal (GI) problems.
| Type of CHD | No. (%) |
|---|---|
| Atrio-ventricular septal defect (AVSD) | 36 (36.0) |
| Ventricular septal defect (VSD) | 35 (35.0) |
| Patent ductus arteriosus (PDA) | 33 (33.0) |
| Atrial septal defect (ASD) | 22 (22.0) |
| Tetralogy of Fallot (TOF) | 9 (9.0) |
| Coarctation of the aorta (COA) | 3 (3.0) |
| Sub aortic web (SAW) | 1 (1.0) |
Thyroid function was evaluated in 70 patients, and 17 (24.27%) had hypothyroidism. GI disorders,such as GI obstruction, imperforated anus, and Hirschsprung disease, were detected in nine (9%) patients (Table 2).
| Non-Cardiac Anomaly | No. (%) |
|---|---|
| Hypothyroidism | 17 (24.27) |
| Gastrointestinal disorder | 9 (9.0) |
| Club foot | 3 (3.0) |
| Genitourinary disorder | 2 (2.0) |
| Leukemia | 1 (1.0) |
| Ophthalmic disorder | 2 (2.0) |
| Dermatological disorder | 1 (1.0) |
| Diabetes mellitus | 1 (1.0) |
| Hearing loss | 1 (1.0) |
5. Discussion
In this study, the male to female ratio was approximately one (48%:52%). In another similar study, 43.90% of patients were male and 56.09% were female (17). The mean birth weight of the patients was 2744.88 grams, which was in the normal range. Of the patients studied, 70% had a birth weight in the range of 2500 to 3500 grams, and about 28% of them were SGA. In the general population, 10% of newborns had a birth weight of less than 2500 grams. This difference could be due to developmental delays during the fetal period in patients with DS. Only 2% of patients were LGA, which is less than the general population.
This study showed that 62% of mothers were younger than 35 years, which is due to a higher prevalence of reproduction at younger ages. According to some studies, paternal age, in addition to maternal age, also increases the risk of DS (9, 10, 22).
Parents were distant blood relatives in 33 (33%) of the cases, and a familial history of DS was detected in three (3%) of the patients. Akbari conducted a study in which 6.2% of the cases had a familial history of DS (6).
Out of the 100 cases in this study, 61 had genetic assessments, and the results showed that free trisomy was the most common type of DS (98.40%) (This assessment was proposed for every patient, but some parents refused to consent to it because of socioecological, cultural, and economic issues). Akbari estimated the prevalence of free trisomy as 90.70% in DS cases (6). The results of another study by Morris et al. showed that in 29256 cases diagnosed between 1989 and 2009 in England and Wales, nearly 97% of all cases were free trisomy 21, 2.90% were contributory trisomy 21, 0.3% were double or triple aneuploidies, and 1% were mosaics (23).
In our patients, AVSD was the most frequent CHD, followed by VSD. The frequency of PDA (33%) was significantly higher in our study than in others (6, 7). In the Akbari study conducted in Iran, prevalences for the most common CHDs were as follows: AVSD, 50%; VSD, 21.8%; ASD, 18.7%; and TOF, 6.2% (6). In a study by Martinez-Quintana, AVSD was the most frequent CHD (63%) followed by VSD (26%), and Eisenmenger was detected in 21% of cases (7).
This study found that hypothyroidism and gastrointestinal abnormalities were the most common non-cardiac anomalies. According to other studies, hypothyroidism affects 15 - 20% of Down syndrome patients and 0.3% of the general population (8). In this study, about 22.3% of patients had hypothyroidism. This difference could be due to the high prevalence of hypothyroidism in our province. This means that thyroid function in a more diverse sample of patients with Down syndrome must be evaluated.
Parents were third degree blood relatives in 33 (33%) of cases, which is a high rate, and a familial history of DS was detected in three (3%) of the patients. Therefore, non-random mating is an important issue in developing countries that needs more attention, and in Iran, genetic counseling should be recommended to every couple before marriage.
Although chromosomal analysis was proposed for every patient, some parents refused because of socioecological, cultural, and economic issues. Therefore, it seems that economic and social support is necessary for this group.
Management of Down syndrome requires an organized approach to ongoing evaluation and monitoring for associated abnormalities and prevention of common disorders such as cardiac problems, thyroid and GI disorders, and disorders of the central nervous system. To reach this goal, different medical specialists should be available to DS patients within the clinical setting, including geneticists, pediatricians, cardiologists, and endocrinologists. This comprehensive approach will help clinicians manage DS more efficiently.