1. Introduction
Topical corticosteroids are widely used to treat various skin and inflammatory disorders. However, topical corticosteroid use may lead to several adverse effects. Absorption through the skin may lead to Cushing’s syndrome with suppression of the hypothalamic-pituitary-adrenal axis (1). Decani et al. (2) reported on the cases of five patients with systemic side effects following the use of topical corticosteroids, whereas 43 cases with exogenous Cushing’s syndrome over 35 years, two of whom died from severe cytomegalovirus infection, have been reviewed and reported by Tempark et al. (3). The use of clobetasol propionate, a potent corticosteroid, has not been adequately assessed in children, and is therefore not recommended in those aged under 12 years (3).
2. Case Presentation
Herein, the cases of three children and a 25-year-old man with a history of topical steroid usage (clobetasol propionate) for diaper rash and dermatitis, respectively, are described. All four cases developed Cushing’s syndrome, with life threatening consequences in one case. After a complete physical examination, the children’s height and weight were plotted on CDC 2000 US curves. Standard deviation scores were calculated as patient height minus 50th percentile height/standard deviation for age and sex. Cortisol and adrenocorticotropic hormone (ACTH) were measured by electrochemiluminescence immunoassay (Cobas e411, Roche Diagnostics, Mannheim, Germany).
2.1. Case 1
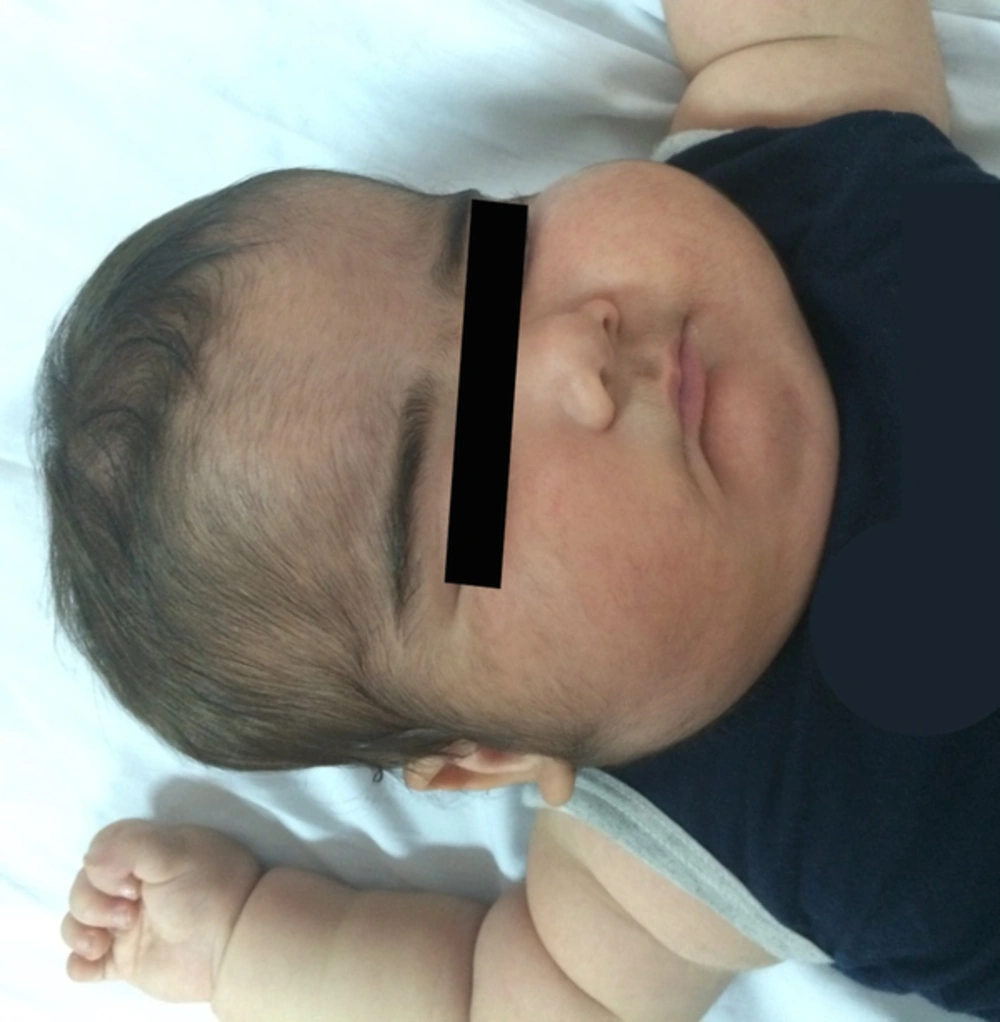
An 11-month-old boy was referred to the endocrine clinic because of irritability and obesity. He was born through normal vaginal delivery at 38 weeks gestation, with a birth weight of 3,250 kg from healthy, unrelated parents. He had an 8-month history of diaper rash and his mother had applied clobetasol propionate ointment 0.05%, 6 – 7 times a day, for 4 months, and continued with 3 – 4 daily applications for a further 4 months. On physical examination, he had a blood pressure of 170/100 mmHg, a pulse rate of 90 beats/min, body temperature of 37°C, and a respiratory rate of 25 breaths/min. A cushingoid appearance, moon face, telangiectasia on the cheeks, hypertrichosis and several ecchymotic skin lesions were found on the face and limbs (Figure 1). He had a diaper rash with erythematous papules, suggestive of candidiasis. His body weight and height were 8.6 kg (between 10th and 25th percentile in the CDC 2000 growth curves) and 64 cm (under 3rd percentile, –4 standard deviation scores), respectively, and he had a head circumference of 45 cm (between 25th and 50th percentile).
Blood tests revealed low levels of cortisol and ACTH in the 08:00 AM (Table 1). Other laboratory evaluations were as follows: hemoglobin, 12.4 g/dL; white blood cell, 12,000/mm3; serum creatinine, 0.5 mg/dL; serum sodium, 136 mEq/L; serum potassium, 4.3 mEq/L; and fasting plasma glucose, 114 mg/dL (returned to normal on follow-up). The lipid profile showed higher than normal cholesterol (241 mg/dL) and low-density lipoprotein (164 mg/dL) levels for age and sex, both of which returned to normal on follow-up. Triglyceride and high-density lipoprotein were both normal and 24-h urine-free cortisol was 10 g (normal range, 10 – 100). Serum aldosterone was 114 pg/mL (25 – 313) and renin was elevated (> 500 ng/mL). Echocardiogram, retinal examination, and Doppler sonography of the kidneys were normal. Sonography of the adrenal glands and computerized tomography of the abdomen and pelvis were normal, without any space-occupying lesions, although mild fatty liver was reported.
| Cortisol (µg/dL) | Adrenocorticotropic Hormone (pg/mL) | |
|---|---|---|
| Case 1 | ||
| Age, mo | ||
| 11 | 0.72 | 1.5 |
| Case 2 | ||
| Age,mo | ||
| 4 | 3.3 | 18.7 |
| Case 3 | ||
| Age, y | ||
| 3.5 | 2.14 | 1.83 |
| Case 4 | ||
| Age, y | ||
| 25 | 0.5 | < 1 |
| Reference values | 5 – 16 | 7.2 – 63 |
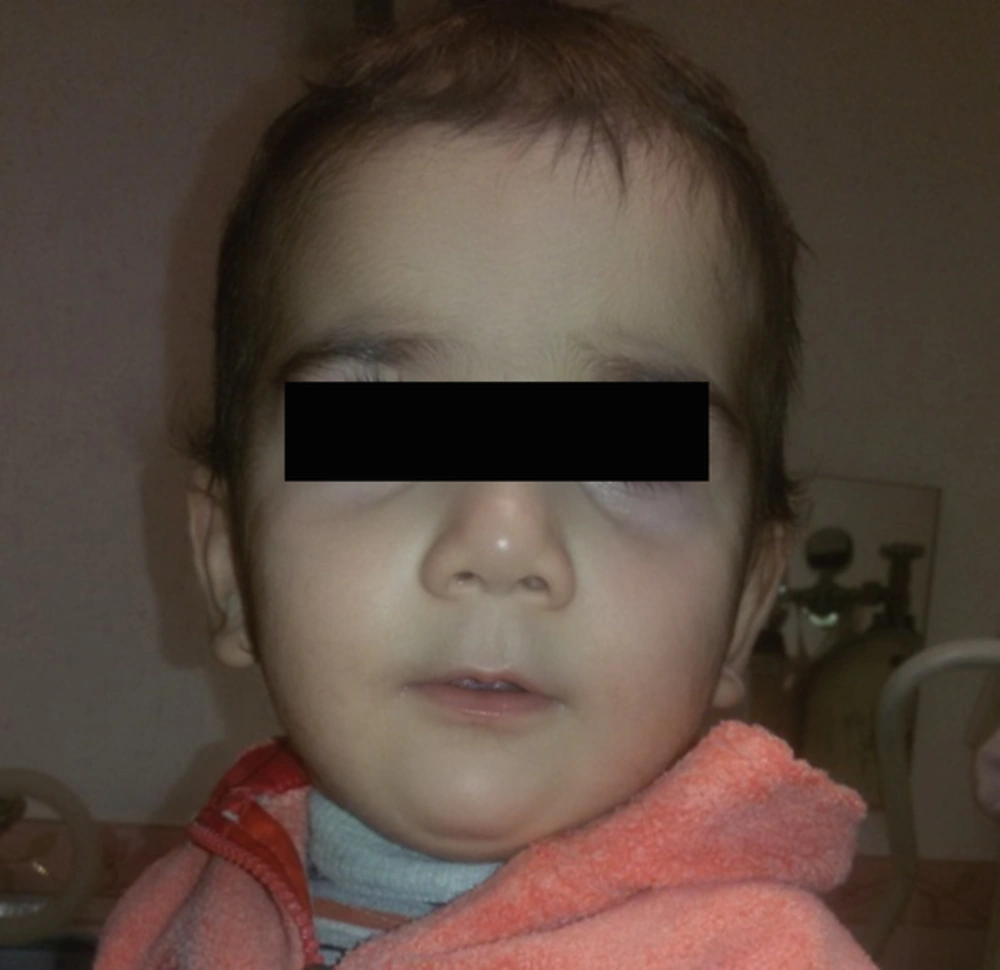
Topical steroid application was stopped, and the patient was administered a physiological dose of hydrocortisone and gradually tapered. Topical Clotrimazole was applied for skin lesions and a stress dose of hydrocortisone was suggested during illness, fever, and surgery to prevent adrenal crisis. Hypertension was controlled with intravenous labetalol, and then changed to enalapril. Figure 2 reveals the patient’s face after 4 months of discontinuation of hydrocortisone.
2.2. Case 2
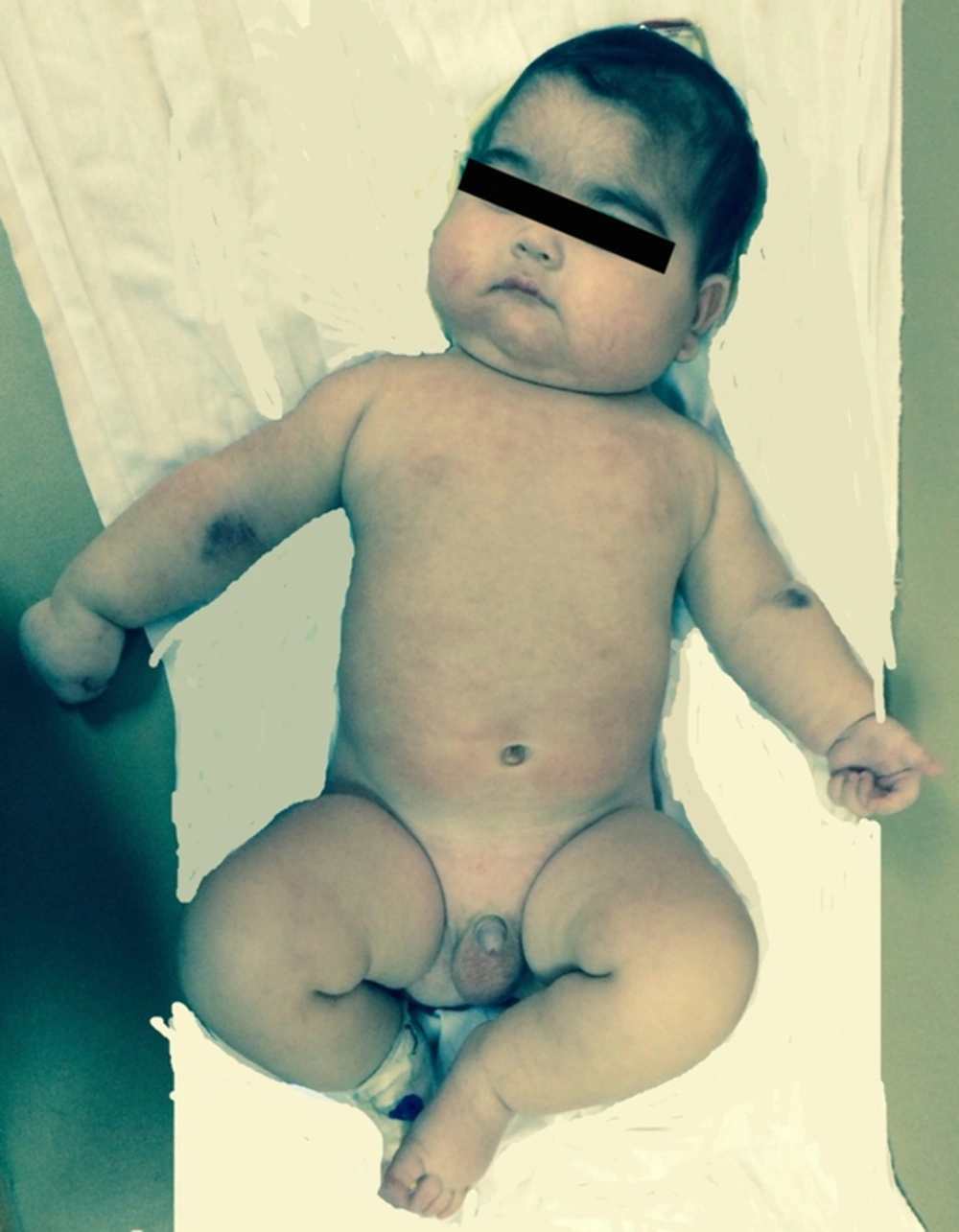
The patient was a 4-month-old boy referred due to a cushingoid face (Figure 3). He was born full term from unrelated, healthy parents by cesarean delivery. On physical examination, his weight and height were normal, at 6.650 kg (between 25th and 50th percentile) and 63 cm (on 50th percentile), although cushingoid features were noticed. The mother had applied clobetasol cream on the diaper area since birth until 3 months and 1 week. Despite clinical manifestations of Cushing’s syndrome, serum cortisol and ACTH were low (Table 1). Sonography of adrenal glands was normal. Arterial blood gas, cell blood count, 3-hour fasting plasma glucose, and the biochemical profile were all normal. The orders for stress were suggested for him and hydrocortisone was not administered.
2.3. Case 3
This patient was a 3.5-year-old boy with clinical features of Cushing’s syndrome consisting of a round plethoric face, a buffalo hump, and abdominal obesity, which appeared at 2 years of age according to his medical report. He had a history of prolonged clobetasol use on the diaper area. After discontinuation of clobetasol, hydrocortisone had been started for adrenal insufficiency. Nevertheless, hypertension, commonly accompanying Cushing’s syndrome, continued after clobetasol discontinuation. The child had presented with hypertensive encephalopathy, acute renal failure, and impaired vision, and therefore had been treated with angiotensin-converting enzyme inhibitors. Encephalopathy and renal failure had regressed, but the visual damage was permanent. During renal insufficiency, he had the history of serum sodium and potassium levels of 119 mEq/L and 2.8 mEq/L, respectively, which later returned to normal. His laboratory test results are illustrated in Table 1. A tapering and stress dose schedule was given to the parents.
2.4. Case 4
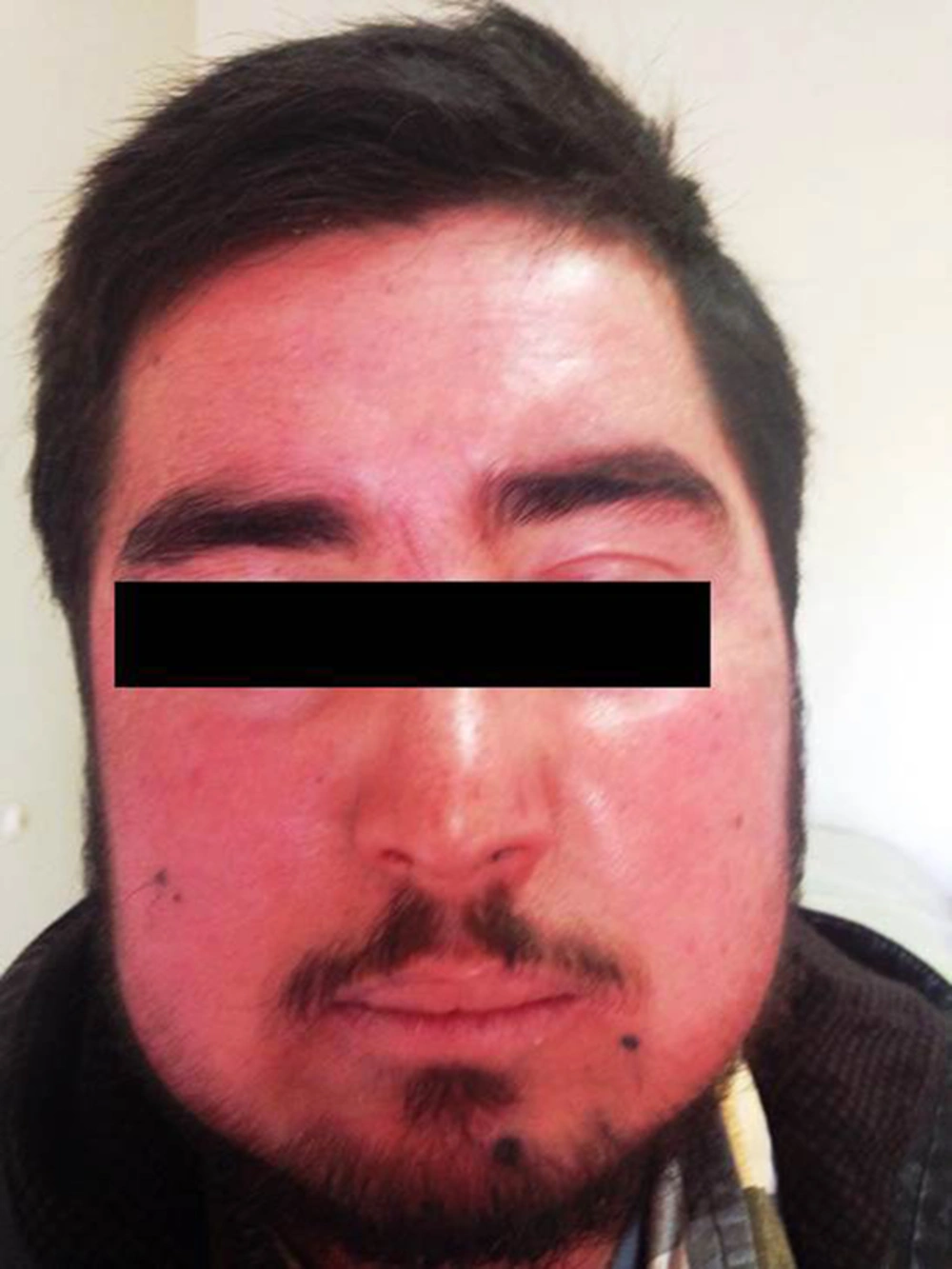
The patient was a 25-year-old man who had gained 9 kg in the past 2 years with associated facial fullness and plethora. He had a history of dry skin and had been treated with topical clobetasol ointment 3 times per week on his hands and face (Figure 4).
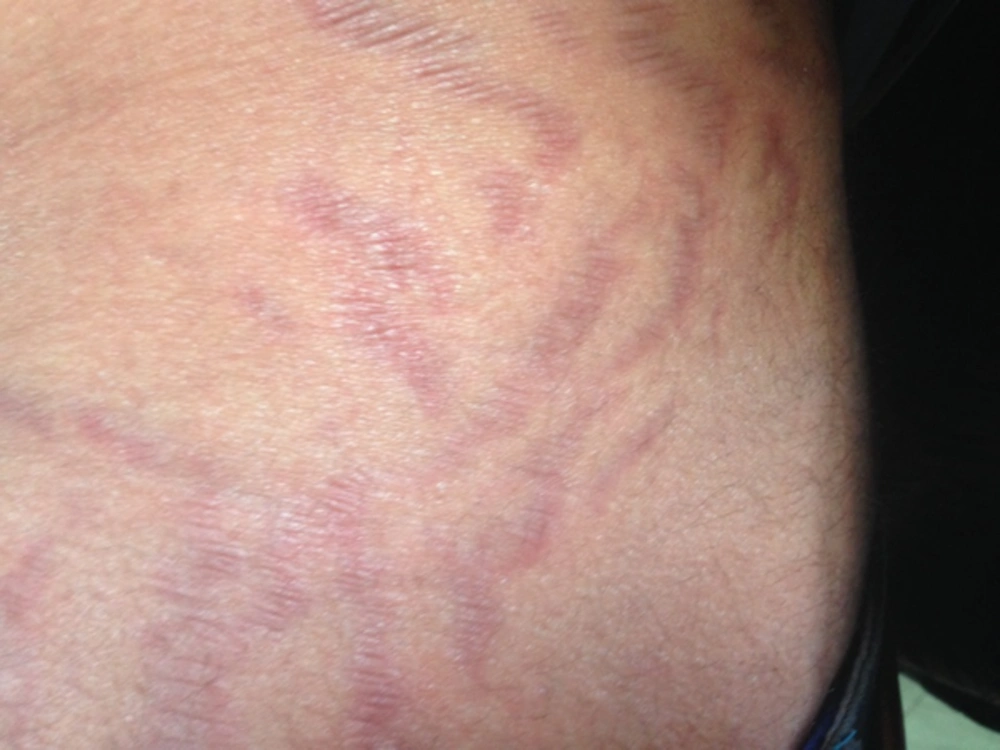
On physical examination, he had a cushingoid-appearance with a blood pressure of 143/95 mmHg and a pulse rate of 72 beats/min. His height and weight were 167 cm and 72.5 kg, respectively. He had substantial supraclavicular and dorsocervical fat accumulation (Buffalo hump) and striae on the lower abdomen, arm, and upper thigh (Figure 5). Laboratory findings were as follows: serum sodium, 145 mEq/L; serum potassium, 3.9 mEq/L; fasting blood sugar, 92 mg/dL; creatinine, 0.8 mg/dL; and 24-hour urine-free cortisol, 2.8 µg. Thyroid function tests were normal. Prednisolone at a dose of 7.5 mg/day, divided in two daily doses, was administered and tapered over 3 months. After a further 3 months, cortisol and ACTH levels returned to normal.
3. Discussion
The current report describes the cases of three children presenting with clinical features of Cushing’s syndrome following prolonged treatment of a diaper rash with clobetasol and that of an adult patient presenting with similar features following clobetasol application on the face and hands. Topical steroids are systemically absorbed and may cause adverse side effects such as iatrogenic Cushing’s syndrome and adrenal suppression due to inhibition of corticotropin-releasing hormone/ACTH secretion (1, 2). Cushing’s syndrome has been reported in both children and adults following the prolonged use of potent corticosteroids as topical cream or oral solutions (2-6). Peppa et al. (7) reported that hypertension is less profound in exogenous, corticosteroid-induced Cushing’s syndrome; nevertheless, in two of the cases herein, hypertension was profound and persisted even after corticosteroid discontinuation and was accompanied by adrenal insufficiency. In one patient, the side effects resulted in life threatening events, hypertensive encephalopathy, renal insufficiency with sodium and potassium wasting, and permanent visual loss. In case 1, aldosterone levels were normal but renin levels were high, indicating that aldosterone levels may have been normal at the expense of high renin levels, which may have also lead to high angiotensin (a potent vasopressor) levels, although this was not measured. Despite adrenal insufficiency and discontinuation of hydrocortisone, hypertension persisted; this is of particular importance in the treatment of pediatric patients for whom blood pressure measurement is difficult due to crying of the child. Clobetasol propionate is a potent steroid (600 – 1000 times more potent compared to hydrocortisone) and is claimed to have a low skin absorption, nevertheless, all of the patients herein used this drug. Even relatively low doses of this class of steroids (2g/day for 2 weeks) have been associated with adrenal gland suppression. (8, 9) Highly vascular areas, such as the ear canals and areas under occlusion such as the diaper area, have been associated with enhanced systemic absorption (8, 9). Differences in individual sensitivity to corticosteroids should also be considered (2). In 1998, Huizenga et al. (10) described a polymorphism in the glucocorticoid receptor gene that was associated with increased receptor sensitivity. N363S mutation has also shown an increase in the therapeutic effect of glucocorticoids (2). Mucocutaneous infections are common during corticosteroid treatment, often occurring early in the therapy and likely being caused by immunosuppression (10, 11). Whenever a cushingoid appearance is accompanied by adrenal insufficiency, the exogenous use of steroids, especially topical creams, should be explored. Hypertension may persist even after cessation of use and may result in life threatening complications. The possible complications of long-term topical steroid applications should be appropriately indicated to the patients.