1. Background
Respiratory distress syndrome (RDS) due to the lack of surfactant is one of the main causes of mortality and morbidity in preterm infants. The risk of this complication is related to gestational age at birth (1-3). The surfactant is produced in the fetal lung tissue during the third trimester of pregnancy and naturally reduces alveolar surface tension, facilitates alveolar expansion, and reduces the possibility of collapse and atelectasis (4). The exogenous surfactant was used for the first time in 1980 to treat RDS in preterm infants. Studies have demonstrated the effectiveness of this treatment in reducing RDS-induced mortality and complications (5). Today, preterm infants at risk of RDS receive an early treatment with surfactant within 2 hours of birth, which improves their survival rate and reduces the likelihood of bronchopulmonary dysplasia (BPD) (6).
Despite the benefits of surfactant administration, complications may also occur after this procedure depending on the administration method used and the success in respiratory management. Occasionally, laryngoscopy and endotracheal intubation fail and may then cause hypoxia, bradycardia, elevated ICP, and respiratory tract trauma (7, 8). Mechanical ventilation may also cause barotrauma and lung damage and thus predisposes the infant to chronic lung disease (CLD). Today, healthcare providers prefer to use noninvasive ventilation techniques for mitigating these complications (9, 10). CPAP is an alternative method to endotracheal intubation that can prevent such complications in infants who are capable of spontaneous breathing, and together with the prenatal administration of corticosteroids, this technique can help reduce the number of infants with clinical symptoms of RDS to a significant degree (11). Nevertheless, these measures are not sufficient per se. Since assisted ventilation before surfactant administration may cause severe lung damage due to low lung compliance, the InsurE (intubation-surfactant-extubation) technique has been proposed to reduce the risk of lung damage as well as the need for mechanical ventilation (10, 12). This method is used in infants on CPAP who show increased symptoms of RDS and require supplemental oxygen. To perform the procedure, infants are intubated after sedation, the surfactant is administered to them through the endotracheal tube, and they are then extubated and placed under respiratory support with CPAP until they reach respiratory stability (13). Birth at less than 30 weeks of gestation, weighing below 750 grams at birth, and having an a/A gradient < 0.44 in the initial arterial blood gases test have been identified as risk factors for INSURE failure (14).
The INSURE may be beneficial in that it helps establish sufficient functional residual capacity (FRC) in preterm infants; yet, intubation and mechanical positive pressure ventilation (even for a relatively short period) can cause complications (10). As a result, the minimally invasive surfactant administration (MIST) technique has been proposed as an alternative, in which, following direct laryngoscopy, the surfactant is slowly administered through a thin tracheal catheter to infants capable of spontaneous breathing and supported by CPAP. This technique does not require the administration of sedatives or the use of positive pressure ventilation and causes less damage to the airways and is well-tolerated by tinier infants (15, 16).
2. Objectives
The present study was conducted to assess the present capacities for performing the MIST technique and to examine its long- and short-term complications and to compare them with the conventional INSURE technique in the treatment of RDS in preterm infants (28 to 34 weeks of gestation).
3. Methods
3.1. Study Design and Patients
The present study examined preterm infants (28 to 34 weeks of gestation) with respiratory distress syndrome (RDS) admitted to the neonatal intensive care unit of Roointan-Arash maternity hospital in Tehran from May 2013 to February 2014. The study was approved by the ethics committee of Tehran University of Medical Sciences. This study is registered with the Iranian registry of clinical trials, IRCT2014080716937N4. Infants with an Apgar score of 4 or less at 5 minutes, those who needed intubation and mechanical ventilation, and those with congenital anomalies or lacking parental consent were excluded from the study.
RDS was diagnosed in the infants based on their need for supplemental oxygen, clinical signs of tachypnea, retractions, and grunting and it was confirmed by a chest X-ray and blood gases test.
Preterm infants who required supplemental oxygen and had good respiratory efforts were put on nasal CPAP. CPAP levels ranged from 5 to 8 cm H2O and FiO2 was adjusted so as to maintain an oxygen saturation of 85% - 92%. Infants requiring FiO2 > 50% and those with respiratory acidosis (pH < 7.2) or prolonged apnea were intubated (17).
Infants with RDS who required FiO2 > 40% to maintain an oxygen saturation (SpO2) in the range of 85% - 92% were randomized to receive surfactant by either the INSURE or the MIST technique.
Intravenous caffeine was administered with a loading dose of 20 mg/kg and continued with a maintenance dose of 5 mg/kg per day.
Before the randomization, informed written consents were obtained from the parents.
3.2. Randomization and Masking
Simple randomization was used in the allocation of participants. In case of multiple pregnancies, each infant was examined separately. Blinding was not performed in any of the stages of the study, from the intervention to the data analysis and the interpretation of the results.
3.3. The INSURE Procedure
The infant was first intubated through the mouth with an endotracheal tube appropriate to his weight and gestational age. A 200 mg/kg dose of surfactant (Curosurf, Chiesi Farmaceutici Group, Parma, Italy) was administered by a feeding tube passed through the tracheal tube over 1 - 3 minutes, during which positive pressure ventilation was applied by a self-inflating bag and the infant was then extubated and placed on nasal CPAP once again. After 12 hours, if FiO2 > 40% was still required to maintain O2 saturation within the range of 85% - 95%, the second dose of surfactant was administered.
The treatment was considered a failure if pH < 7.2, FiO2 > 60%, and PCO2 > 60 mmHg persisted for longer than 2 hours or if apnea occurred, upon which the infant was intubated, and if required, surfactant was administered.
3.4. The MIST Procedure
In this method, a 5-f feeding tube was passed into the trachea through the vocal cords and under direct observation and with no sedatives, using a standard laryngoscope with blade selected based on the infant’s weight. If the catheter failed to pass through within 30 seconds, nasopharyngeal CPAP was used and another attempt was made with the catheter. A 200 mg/kg dose of surfactant (Curosurf, Chiesi Farmaceutici group, Parma, Italy) was then instilled into the infant’s trachea over 1 to 3 minutes or in 3 to 4 stages lasting 15 to 30 seconds. During the procedure, the infant’s SPO2 and heart rate were constantly monitored using pulse-oximetry. If the SPO2 dropped to less than 80% or the heart rate dropped to less than 100 beats/min, the procedure was stopped and FiO2 was increased, followed by PEEP increment. After completing the procedure, the infant’s stomach was suctioned to ensure that surfactant had entered the lung. The catheter was then removed, CPAP was resumed, and FiO2 then gradually reduced. If apnea persisted, PPV was administered with Ambu-bag and mask. The criteria for the administration of the second dose of surfactant as well as for the need for intubation and mechanical ventilation were similar to those presented in the INSURE protocol.
The INSURE and MIST techniques were performed by two trained pediatricians.
The primary outcomes of the study, including the need for intubation during the first 72 hours and afterwards, the incidence of pulmonary hemorrhage, pneumothorax, patent ductus arteriosus requiring medical or surgical treatment, intraventricular grade > 2, and the secondary outcomes, including retinopathy of prematurity greater than stage 2, necrotizing enterocolitis of stage 2 or greater, sepsis, bronchopulmonary dysplasia (oxygen dependence at 36 weeks of postmenstrual age) and death, were evaluated and compared between the two groups.
3.5. Statistical Analysis
Previous studies have shown that almost 45% of the infants in the INSURE group and 22% in the MIST group require intubation during the first 72 hours of life. To demonstrate this hypothesis, a sample size of 26 was estimated for each group given a statistical power of 80%. The data obtained were analyzed in SPSS-19 (IBM Corporation, Armonk, NY). The mean and standard deviation were used for the descriptive analysis of the data and the median and range for the other data. In the case of the normal distribution of the dependent variable, the t-test was used for quantitative variables and the chi-squared test for qualitative variables, and if required, Fisher’s exact test was used, as well. In the case of non-normal distribution of the data, non-parametric tests such as Mann-Whitney’s U test were used. The level of statistical significance was set at P < 0.05.
4. Results
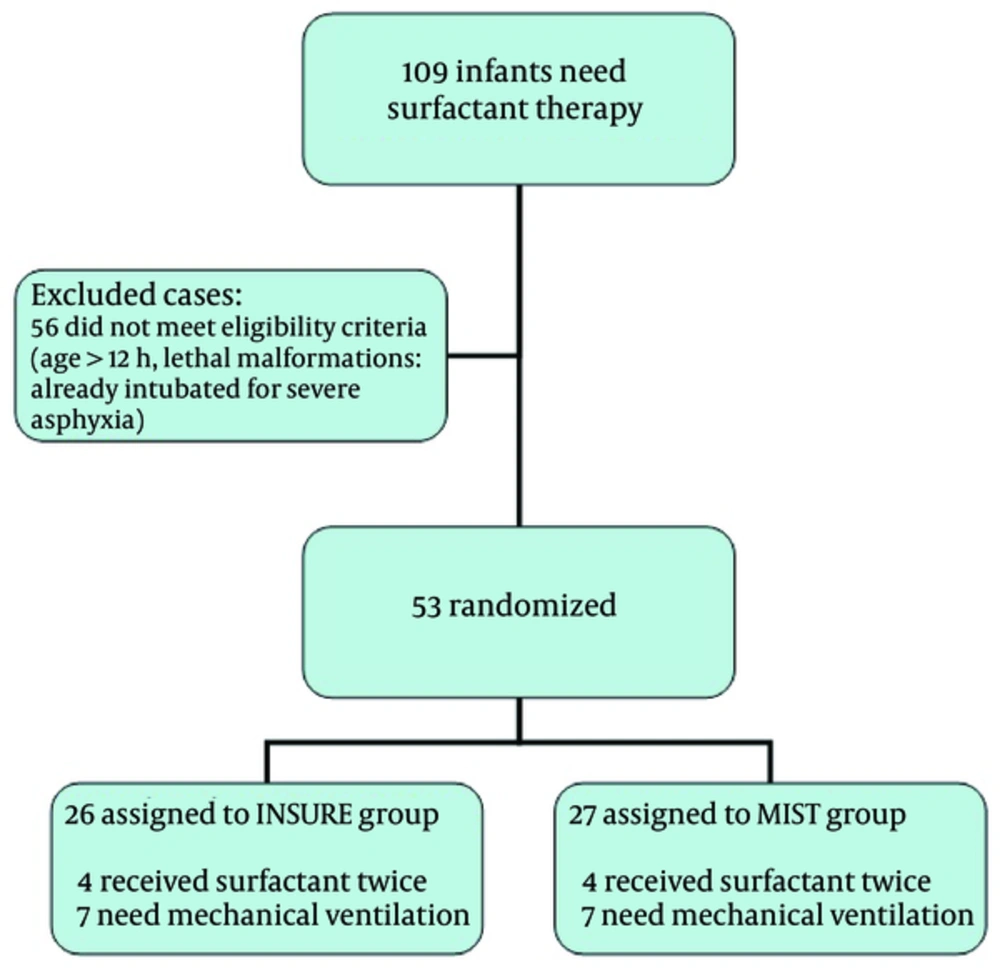
Figure 1 presents the trial profile.
Given the respiratory status and surfactant requirements, infants below 34 weeks of gestation were included in the study. Of the 26 infants treated with the INSURE technique, 4 required a second dose of surfactant and 7 needed mechanical ventilation in the first 72 hours of life. Of the 27 infants treated with the MIST technique, 2 required a second dose of surfactant and 8 needed mechanical ventilation in the first 72 hours of life.
A total of 109 preterm infants with a gestational age less than 34 weeks born with RDS received surfactant, 53 of whom met the study inclusion criteria. About 49% of the infants received surfactant by the INSURE and 51% by the MIST technique.
No differences were observed between the infants’ clinical features in the randomization stage (Table 1).
| Variable | INSURE | MIST | P Value |
|---|---|---|---|
| Gestational age (w) | 31.9 (± 1.5)a | 32.6 (± 1.1)a | 0.063 |
| Birth weight (g) | 1910.0 (± 433.0)a | 1791.9(± 554.3)a | 0.39 |
| Male, n (%) | 11 (42.3%) | 16 (59.3%) | 0.27 |
| Apgar 1 minute | 6.7 (4 - 9)b | 6.7 (3 - 9)b | 0.95 |
| Apgar 5 minutes | 8.3 (7 - 10)b | 8.2 (4 - 10)b | 0.95 |
| Antenatal steroids, n (%) | 15 (57.7%) | 14 (51.9%) | 0.67 |
| C-section, n (%) | 24 (92.3%) | 25 (92.6%) | 1.00 |
| Time interval from birth to surfactant administration (h) | 3.4 (± 2.2)a | 3.0 (± 2.0)a | 0.45 |
| FiO2 prior to surfactant administration (%) | 79.2 (± 11.1)a | 74.4 (± 18.6)a | 0.36 |
| Maternal age (y) | 28.6 (± 4.4)a | 26.8 (± 4.7)a | 0.15 |
aMean (SD).
bMedian (range).
The MIST group received surfactant at 3.0 ± 2.0 hours of birth and the INSURE group at 3.4 ± 2.2 (P = 0.45).
In the MIST group, the first catheterization failed in 7 of the infants (25.9%) and the procedure had to, therefore, be repeated. A second dose of surfactant was required in 4 infants (15.4%) in the INSURE group and in 2 infants (7.4%) in the MIST group.
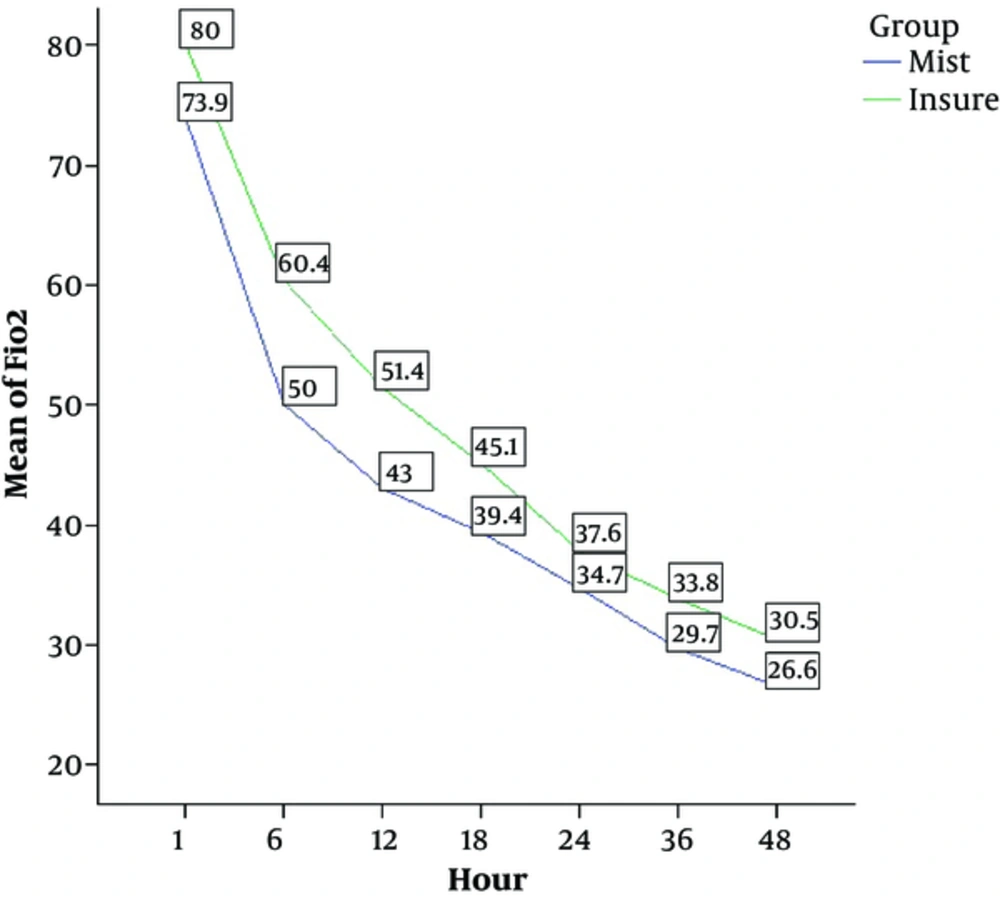
The administration of surfactant was followed by a reduction in FiO2 in both the groups; within four hours of the administration, the need for supplemental oxygen reduced significantly in both the groups; the mean FiO2 was 38.3% in the MIST group and 36.8% in the INSURE group. Overall, FiO2 was 42.5 ± 19.6% (95%CI; 38.15 - 46.8) in the MIST group and 48.4 ± 21.6% (95% CI; 44.03 - 52.8) in the INSURE group, making for a significant intergroup difference (P = 0.009).
Figure 2 shows the oxygen requirement in the first 48 hours of the procedure in both groups.
A total of 15 infants (28.3%) required intubation in the first 72 hours, 7 (26.9%) of whom were in the INSURE group and 8 (29.6%) in the MIST group (P = 0.827). The duration of NICU stay was shorter in the MIST group than in the INSURE group (7.3 ± 7.2 vs. 10.4 ± 9 days), P = 0.81).
There were no significant differences between the two groups in relation to the secondary outcomes.
In the MIST group, one infant (3.7%) with a birth weight of 1150 grams and a gestational age of 31 weeks died on the 7thday of birth due to sepsis.
Table 2 presents the primary and secondary outcomes in the two groups
| Variable | INSURE | MIST | P Value |
|---|---|---|---|
| Primary Outcomes | |||
| Second dose surfactant, n (%) | 4 (15.4%) | 2 (7.4%) | 0.42 |
| Coughing or gagging, n (%) | 9 (34.6%) | 10 (37.0%) | 0.55 |
| Surfactant reflux, n (%) | 6 (23.1%) | 6 (22.2%) | 0.6 |
| Bradycardia (< 100 bpm), n (%) | 7 (26.9%) | 3 (11.1%) | 0.13 |
| Apnea | 2 (7.7%) | 3 (11.1%) | 0.37 |
| Intubated in first 72 hours of life, n (%) | 7 (26.9%) | 8 (29.6%) | 0.827 |
| Duration of oxygen consumption (h) | 71.9 (70.6) | 79.1 (48.6) | 0.15 |
| Pneumothorax, n (%) | 1 (3.8%) | 0 (0%) | 0.49 |
| Secondary Outcomes | |||
| Pulmonary hemorrhage, n (%) | 1 (3.8%) | 0 (0%) | 0.49 |
| Intra ventricular hemorrhage (> grade 2), n (%) | 1 (3.8%) | 0 (0%) | 0.49 |
| Patient ductus arteriosus, n (%) | 3 (11.5%) | 3 (11.1%) | 1.000 |
| Broncho pulmonary dysplasia, n (%) | 1 (3.8%) | 0 (0%) | 0.49 |
| Retinopathy of prematurity (> stage 2), n (%) | 0 (0%) | 0 (0%) | 1.000 |
| Necrotizing enterocolitis, n (%) | 1 (3.8%) | 0 (0%) | 0.49 |
| Length of stay in NICU (D) | 9 (10.4) | 7.3 (7.2) | 0.81 |
| Death, n (%) | 0 (0%) | 1(3.7%) | 0.98 |
5. Discussion
Studies conducted in the 1980’s and 1990’s on preterm infants at risk of RDS showed surfactant administration (of animal origins) after birth reduces mortalities and improves survival rate without the development of chronic lung disease complications (18). Some studies show that up to 80% of preterm infants with moderate to severe RDS end up requiring intubation, and this rate is inversely related to gestational age (19). The INSURE technique for administering surfactant reduced the duration of the need for intubation and mechanical ventilation in preterm infants. Nevertheless, this method failed to fully prevent the incidence of intubation complications (20). Verder et al. first published the results of their study on surfactant administration through a thin catheter in infants capable of spontaneous breathing in 1992 (21). However, this technique was first used as a treatment option in 2001 and the first serious study on the feasibility of this technique and its patient prognosis was published in 2007 (22, 23).
This technique appears to quickly improve infants ’physiological conditions and reduce their need for FiO2 and mechanical ventilation and their duration of oxygen treatment (17).
The present study showed a 75% success rate for the first attempt at catheterization using the MIST technique. The 25% failure rate can be explained by the operators’ lack of experience with this method. Other studies also showed the need for repeating catheterization in about 20% of the cases (17, 22).
Meanwhile, since no sedatives are required for this technique, there will be no need for long post-procedure respiratory support. Coughing and gagging were observed in the MIST group in more than one-third of the infants as the most prevalent complication. Other studies reported the prevalence of this complication between 11% and 32% (22, 24). In the present study, intubation was performed with no sedatives; as a result, one-third of the infants developed this complication in the INSURE group, which is consistent with the results obtained by Al Ethawi (24), while Mirnia (25) reported this complication in only 3% of the infants following sedation and intubation.
Surfactant reflux was observed in almost 22.2% of the cases examined in the present study, which is consistent with the results obtained by Kanmaz (22) and Dargaville (17) upon using the MIST technique in preterm infants (29 - 32 weeks of gestation). In the study by Dargaville et al., one-third of preterm infants (25 - 28 weeks of gestation) developed this complication upon using the MIST technique (17). In the present study, this complication was reported in nearly 23.1% of the cases upon the administration of surfactant using the INSURE method.
In the present study, almost 10% of the infants in the MIST group developed bradycardia less than 100 bpm for over 10 seconds. Different studies have reported the prevalence of this complication as 17% to 44% (17, 22, 24).
In the study conducted by Kribs (23), where atropine was administered prior to catheterization, this complication was reported as 7.4%, which is lower than the rate in the present study. This complication was reported in about 25% of the cases in the present study following the administration of surfactant using the INSURE method. This self-limiting complication tends to occur due to stimulations during the attempts to see the vocal cords and is often relieved after pausing the procedure for a few minutes (17).
In the present study, both groups received 200 mg/kg of Curosurf, and about 15% in the INSURE group and 7.5% in the MIST group required a second dose of surfactant. In the study by Kanmaz (22), treatment began with an initial 100 mg/kg dose of Curosurf, and an almost equal percentage of both groups (about 20%) required the second administration of surfactant. In the study by Aguar (16), the infants in the MIST group received a 100 mg/kg dose of Curosurf and those in the INSURE group received a 200 mg/kg dose, and about 36% of the MIST group and only 6.5% of the INSURE group needed the second administration of surfactant. The initial dose of surfactant appears to dictate the need for a second administration regardless of the technique used.
Four hours after the administration of surfactant, the need for supplemental oxygen reduced in both groups by about 20%, which is consistent with the results obtained in other studies (22, 24). These results indicate the good and immediate effects of both techniques in reducing the need for supplemental oxygen due to adequate exogenous surfactant reaching the respiratory alveoli and eliminating underlying atelectasis in patients receiving respiratory support with CPAP.
Auxiliary oxygen was administered nearly 80 hours to the infants in the MIST group and 70 hours in the INSURE group. Unlike the results obtained in the present study, most other studies (17, 22, 24) found the mean oxygen requirement to be relatively greater in the INSURE group, with the difference being significant only in the study by Dargaville (17), in which FiO2 was set to maintain SpO2 between 88% and 92%. In other studies, including in the present one, FiO2 was adjusted to maintain SpO2 between 85% and 92%. The administration of surfactant appears to cause a more immediate and effective lung tissue function correction with the MIST technique, due to its ability to correct atelectasis.
Nevertheless, in terms of the duration of time required for the administration of oxygen, the difference is negligible, only by considering a minimum acceptable value of 85% for SpO2 compared to in the INSURE technique.
The present study showed that the mean FiO2 was consistently lower in the MIST group than in the INSURE group during the first 48 hours after the treatment. As shown in Figure 2, FiO2 did not change significantly over time from values of 70% - 80% at hour 1 to 25% - 30% at hour 48 in either of the treatment groups. It is therefore not possible to combine all the values together over time, which comprises one of the study limitations. Moreover, since FiO2 was set by the researchers to maintain arterial O2 saturation between 85% and 95% and since the researchers were not blinded to the participants’ group allocation, a potential bias may have occurred with respect to the FiO2 setting, making for another limitation of the study. Furthermore, the infants treated with the MIST technique had a shorter NICU stay by about 2 days compared to the infants in the INSURE group, which is consistent with the results obtained in other studies on infants of various age groups (16, 17).
About 30% of the infants in the MIST group and 25% in the INSURE group required intubation in the first 72 hours after their surfactant administration. These results are consistent with the results obtained in other studies (16, 26). Dargaville (17) also showed that, although preterm infants of 25 - 28 weeks of gestation required significantly less intubation in the MIST group than in the INSURE group in the first 72 hours of life, the difference was not statistically significant in preterm infants of 29 - 32 weeks of gestation.
Bronchopulmonary dysplasia was reported in only 3.8% of the cases in the INSURE group, which is consistent with the results obtained by Dargaville with regard to preterm infants of 29 - 32 weeks of gestation (17). In studies conducted on infants below 28 weeks of gestation, this complication was reported in about 10% to 30% when using the MIST technique and about 20% to 30% with the INSURE technique (17, 22). Although the risk of BPD appears to be lower in infants over 28 weeks of gestation, it should be noted that, in more-preterm infants, the need for intubation and even brief positive pressure ventilation by bagging for surfactant administration could be more damaging when using the INSURE method than with the MIST method. On the other hand, spontaneous breathing during the MIST technique is believed to spread surfactant more uniformly and with less lung injury caused. The incidence of BPD could thus be reduced.
PDA was reported in 11% of the infants in both groups. However, in studies by Gopel and Mirnia, this complication was reported as less than 5% (25, 26), while other studies reported it as about 13% to 36% in the MIST group and about 7% to 63% in the INSURE group (16, 17).
After surfactant administration, a major part of lung atelectasis is resolved, and the oxygenation rate therefore improves, pulmonary vascular resistance decreases, and the likelihood of PDA left-to-right shunt increases, which could justify the development of this complication in infants under treatment (17).
Neonatal morbidities such as pneumothorax, pulmonary hemorrhage, grade II intraventricular hemorrhage, and necrotizing enterocolitis were each observed in only one infant treated by the INSURE method. There were no significant differences between the two groups (P > 0.05). These results are consistent with the results obtained by Kanmaz et al. (22).
None of the patients had retinopathy of prematurity greater than stage II, which is in line with the results obtained by Dargaville for preterm infants of 29 - 32 weeks of gestation (17).
Only one preterm infant at 31 weeks of gestation weighing 1150 grams and treated with the MIST method died due to sepsis on the 7th day of birth. Other studies reported an infant mortality rate below 5% in both groups (16, 17). However, the studies did not discuss the cause of the infants’ death.
Although, in this study, preterm infants of 28 to 34 weeks of gestation were treated with 200 mg/kg of surfactant (Curosurf) and although the favorable effect of this method has been demonstrated, further multi-centric clinical trials are required for determining the best gestational age range and the most appropriate dose of surfactant for this type of treatment.
The present study had many limitations, including a small sample size and the failure to include infants with gestational ages less than 28 weeks. Moreover, since the researchers used only Curosurf, they are unable to comment on other surfactant products.
To conclude, surfactant administration via a thin catheter is a feasible and effective treatment for preterm infants. Nevertheless, further studies are required to confirm the implementation and applicability of this method.