1. Background
Fuel cells are one of the alternative energy sources of the production of electrical energy (1). Biofuel cells (BFC) are a certain type of fuel cells which use biological molecules for the production of electrical energy (2). BFC have advantages including the use of renewable bio-catalysts, the possibility of using a variety of fuels, the ability to operate at low temperatures, mild conditions and physiological pH (3). Among biofuel cells, enzyme-based fuel cells (EBFC) have attracted particular attention (4). Today, various enzymes like alcohol dehydrogenase (ADH) and glucose oxidase and so on have been used to design the enzymatic fuel cells. ADH enzyme reduces NAD+ to NADH during oxidation of ethanol (5). There are many reports on using ADH to design BFC (6-8). In 1997, Palmore et al. used ADH enzyme to design the methanol/O2 biofuel cell (9). In 2008, Sokic-Lazic and Minteer used ADH, aldehyde dehydrogenase (AldDH) and S-acetyl-CoA synthetase with enzymes of the citric acid cycle to oxidize methanol completely (10). In general, EBFC divided into two groups of mediated electron transfer (MET) and direct electron transfer (DET) in term of electron transfer types. In DET, electrons are transferred directly to the electrode surface (11). In MET based EBFC, mediators move the electrons to the electrode surface (12). In ADH-based fuel cell often a mediator used to transfer electrons. Various factors including selection of the appropriate support (electrode materials) and method of enzyme immobilization affect BFC efficiency (13). Carbon cloth is one of the best platforms for effective immobilization of various enzymes. They have a three-dimensional network structure and high thickness as a support for biofuel cell set up (14). One of the key points in studying of NAD+-dependent enzymes is restoration of the NAD+ (15, 16). Because direct oxidation of NADH species in different electrodes done at high potentials (17, 18). The electrocatalysts are commonly used to reduce potential of NADH oxidation (19, 20). Various organic compounds such as methylene green (MG), methylene blue and natural red are used greatly as electrocatalyst (21, 22). Carbon nanotubes (CNTs) is one of the materials that used to recycle the NAD+ on biodevices (23). There are many reports on using CNTs to reduce potential of electrochemical oxidation of NADH (8, 24, 25). Furthermore, CNTs have attracted significant attention to make bioelectrodes due to containing excellent electronic properties, high catalytic activity and biocompatibility. They also cause better enzyme orientation and higher enzyme loading (26-28). There are several methods for the enzyme immobilization (29).Various polymers have been used for enzyme immobilization in EBFC (30, 31). PAMAM dendrimer is one of the polymers that has been widely used for immobilization of enzymes on the electrode surface in recent years (32-34). PAMAM dendrimer is a kind of branched polymers that have different features including large uniformity, narrow molecular weight distribution and highly functionalized terminal surface (35). PAMAM dendrimer due to the presence of end groups keep the enzyme on its surface, also its porous and uniform structure maintains the activity of the enzyme (36). G4 PAMAM dendrimer is the smallest spheroid generation of denderimers with interior spaces (37). Astruc and his colleagues confirmed electrochemical reversibility of redox system terminated dendrimers. They introduced for their phenomenon, electron hopping as proposed mechanism for electron transfer of dendrimer modified electrode (37). The Albery theory is applied to analyze the experimental cyclic voltammetry data in order to extract kinetic parameters of the reaction (38).
2. Methods
2.1. Materials
Alcohol dehydrogenase (ADH: E.C. 1.1.1.1) from Saccharomyces cerevisiae (lyophilized powder, 433 Units mg-1), ncotinamide adenine dinucleotide hydrate (NAD+), polyamidoamine generation 4 dendrimer (PAMAM) and methylene green (MG) and multiwalled carbon nanotubes (MWCNTs) purchased from Sigma-Aldrich (U.S.A). Sodium tetraborate (Na2B4O7), sodium nitrate (NaNO3), Sodium phosphate dibasic (Na2HPO4), Sodium phosphate monobasic monohydrate (NaH2PO4. H2O) and Ethanol were purchased from Merck (Germany). Carbon cloth was purchased from Azar Electrode Company (Iran). All solutions were prepared with deionized water and stored at 4°C when not in use.
2.2. Bioanode Preparation
First, a layer of methylene green was electropolymerized by electrochemically cycling on carbon cloth with area of 1 cm × 1 cm in a solution of 0.4 mmol-1 MG and 100 mmol-1 sodium nitrate and 10 mmol-1 sodium tetraborate in the range of -0.3 V to 1.3 V vs. Ag/AgCl with scan rate of 0.05 V s-1 (39). Then, MG film was washed slowly with double distilled water and dried for 24 hours at room temperature. In the next step, 50 µL of commercial PAMAM solution was dropped on poly methylene green (PMG) modified carbon cloth. After drying PAMAM, 50 µl of MWCNTs was deposited on the electrode surface. Then, modified electrode (carbon cloth/PMG/PAMAM/MWCNTs) was allowed to dry completely. Finally, 50 µL of solution enzyme/NAD+ with concentration of 1 mgml-1 enzyme and 1.9 mM NAD+ was casted on the prepared electrode. The modified carbon cloth by PMG/PAMAM/MWCNTs/ADH was used as a bioanode.
2.3. Electrochemical Measurements
All electrochemical measurements of modified carbon cloth were done in a three-electrode cell chamber (Autolab). In this cell, the modified carbon cloth by PMG/PAMAM/MWCNTs/ADH was used as a working electrode. Chamber cell contained 100 mM phosphate buffer (pH 7.4), 1.9 mM NAD+ and various concentrations of ethanol. All the tests have been done at room temperature.
2.4. Derivation Kinetic Parameters from Cyclic Voltammetry
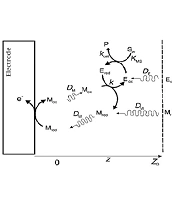
The following scheme shows the homogeneous system that considered by Albery et al. (40). The reactions occurring in solution are:
At the electrode surface:
In this reaction scheme, S and P are substrate and product. Eox and Ered are the oxidized and reduced forms of the enzyme, and Mox and Mred are the oxidized and reduced forms of the mediator (Figure 1).
Albery et al. derived solutions to extract kinetic data from experimental results for different limiting cases (40).
We used the model proposed (case I) by Albery for mediator-enzyme limited kinetics (40).
The amperometric response is given by Equation 1.
In the above equation mΣ, eΣ, k, A, F and n are the concentration of mediator, the enzyme concentration, the rate constant of the reaction between the enzyme and the mediator, the electrode area, faraday constant and number of electrons transferred respectively (38).
Albery et al. also wrote diffusion–reaction equations for enzyme-substrate limited kinetics (cases VI) (40).
The enzyme–substrate kinetics can be calculated from plot of the catalytic current against the substrate concentration. The number of electrons transmitted by the MG mediator is n = 2 (38).
3. Results and Discussion
3.1. Cyclic Voltammetry (CV) Characterization
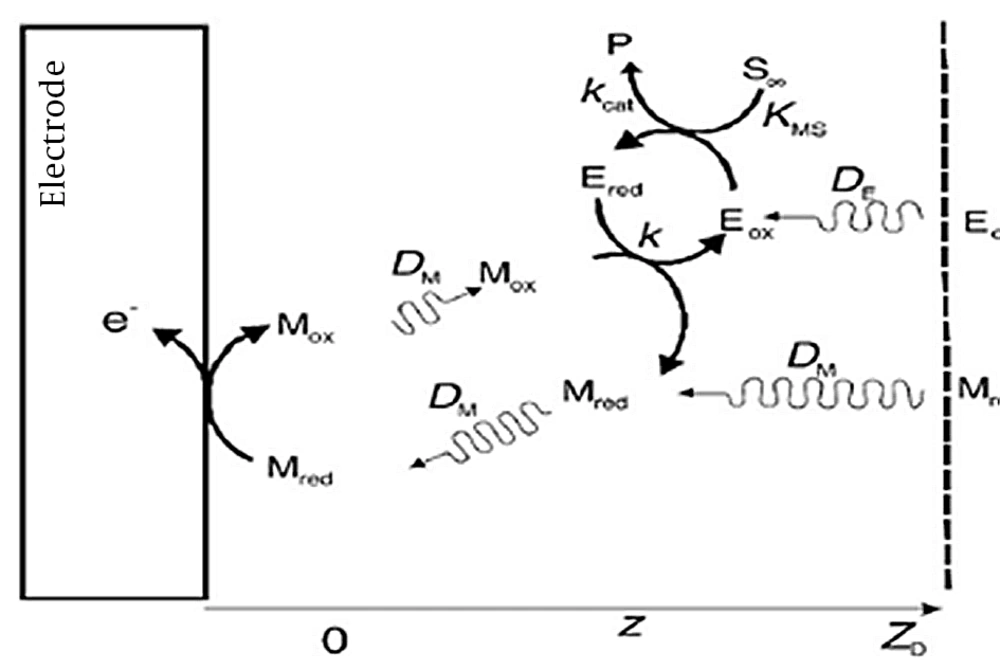
Figure 2 shows the anodic current of the prepared bioanode (carbon cloth/PMG/PAMAM/MWCNTs/ADH) against different concentrations of ethanol in the range of -1 V to +1 V vs. Ag/AgCl in 100 mM phosphate buffer (pH = 7.4), with scan rate of 0.001 V s-1 at 25°C.
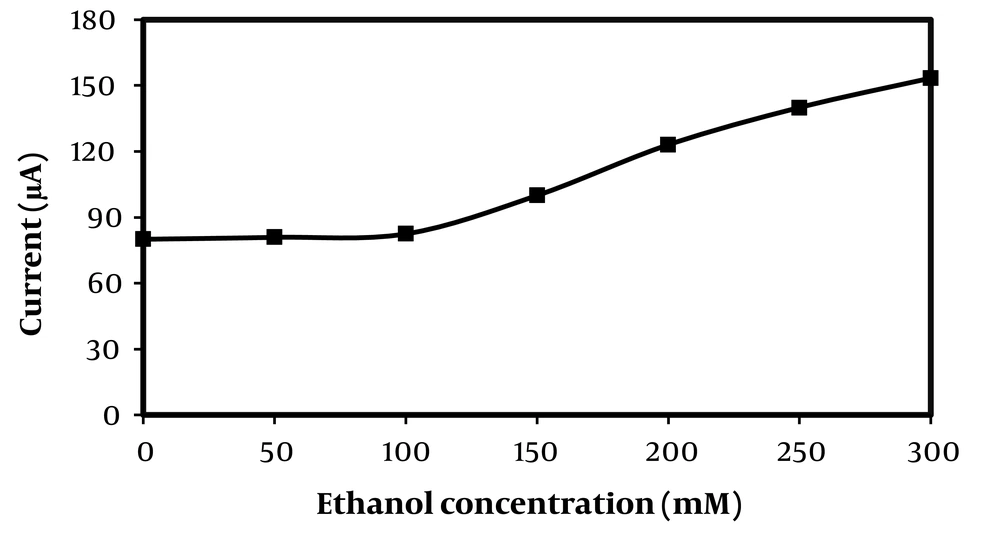
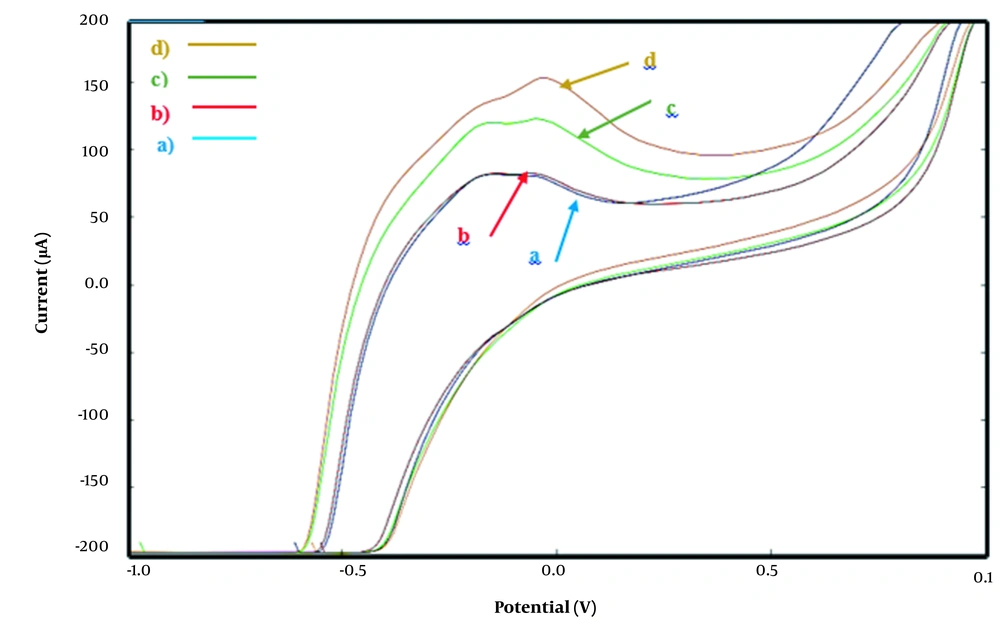
Figure 3 shows the cyclic voltammogram of carbon cloth/PMG/PAMAM/MWCNTs/ADH in 100 mM phosphate buffer in absence of NAD+ and ethanol at scan rate of 0.001 V s-1. As it is shown two anodic peaks at -0.17 V and -0.04 V (which corresponds to the performance of electrochemical NAD+ by PMG and MCNTs) and a weak cathodic peak is exist (blue line). A pair of anodic peaks corresponded to the NAD+/NADH redox process is observed in the presence of ethanol. NAD+ is efficiently reduced with higher potential in the presence of ADH. Enhancement in anodic peak current in the presence of ethanol at carbon cloth/PMG/PAMAM/MWCNTs/ADH suggest that ADH shows electrocatalytic effect for ethanol oxidation. By increasing the concentration of ethanol (from 100 mM to 300 mM) current of anodic peak in potential of 0.25 V is increased more than anodic peak in potential of -0.18 V that represents the increase in NADH electrocatalytic oxidation by the ADH (orange line).
Cyclic voltammogram of carbon cloth/PMG/PAMAM/MWCNTs/ADH electrode in 100 mM phosphate buffer (pH = 7.4), between -1.0 V to + 1.0 V vs. Ag/AgCl, at scan rate of 0.001V s-1 and temperature of 25°C, in absence of ethanol (blue) and in presence of ethanol 100 mM (red line), 200 mM (green line) and 300 mM (orange line).
3.2. Kinetic Data
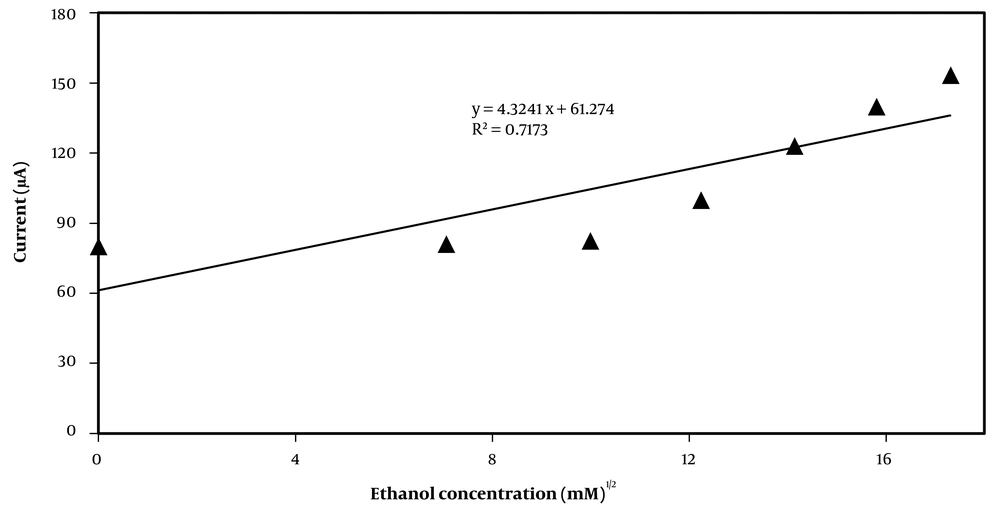
The KMS and kcat are calculated from a plot of the anodic current against ethanol concentration (Figure 2). Currents were taken from the plateau currents in the cyclic voltammograms at 0.25 V and scan rate of 0.001V s-1.
KMS is equal to the substrate concentration at which the current is half of the maximum current. For when the concentration of substrate is very low (s∞ << KMS), anodic current has a linear relationship to the square root of the substrate concentration (38). As a result, Equation 2 is converted to the following equation.
Thus a plot of anodic current against the square root of the substrate concentration (s1/2) shown in Figure 4.
From plot of anodic current against the square root of the substrate concentration as well as can be calculated the rate constant kcat from current in the saturated region, IMAX, when (s∞ >> KMS).
In this study, increasing the concentration of substrate gradually leads to an increase in the amount of anodic current to its maximum.
Table 1 shows the kinetic parameters, kcat, KMS, and k for ADH kinetics mediated by the soluble MG by using analytical equations that have discussed.
| Parameters | Equation 1 | Equation 2 | Equation 4 |
|---|---|---|---|
| KMS (mM) | 4.4 | ||
| kcat (s-1) | 1.6 ± 0.1 | ||
| K × 105 (M-1 s-1) | 1.9 ± 0.1 | ||
| kcat/KMS × 102 ( M-1 s-1) | 3.77 ± 0.3 |
The average concentration of ADH (Γmol cm-2) on the surface of modified carbon cloth calculated from the formula of Q = nFAΓ, where Q, A, F, and n are the charge consumed (C), the electrode area (cm2), F the faraday constant (sAmol-1), and the number of transferred electrons respectively. The immobilized ADH concentration on the modified electrode was estimated approximately 4.152 × 10-6 mol cm-2 (n = 2).
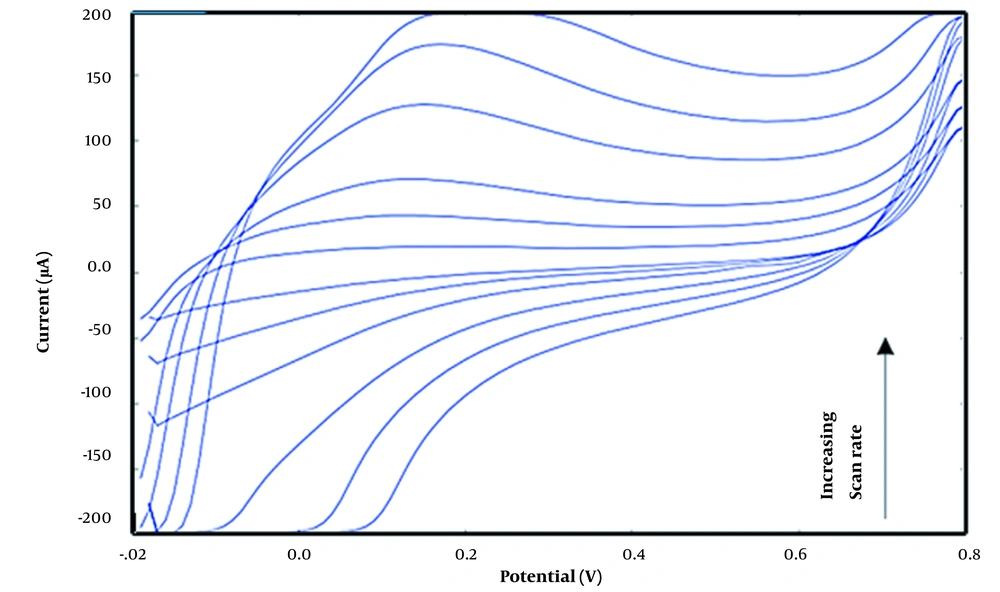
3.3. Effect of Scan Rate
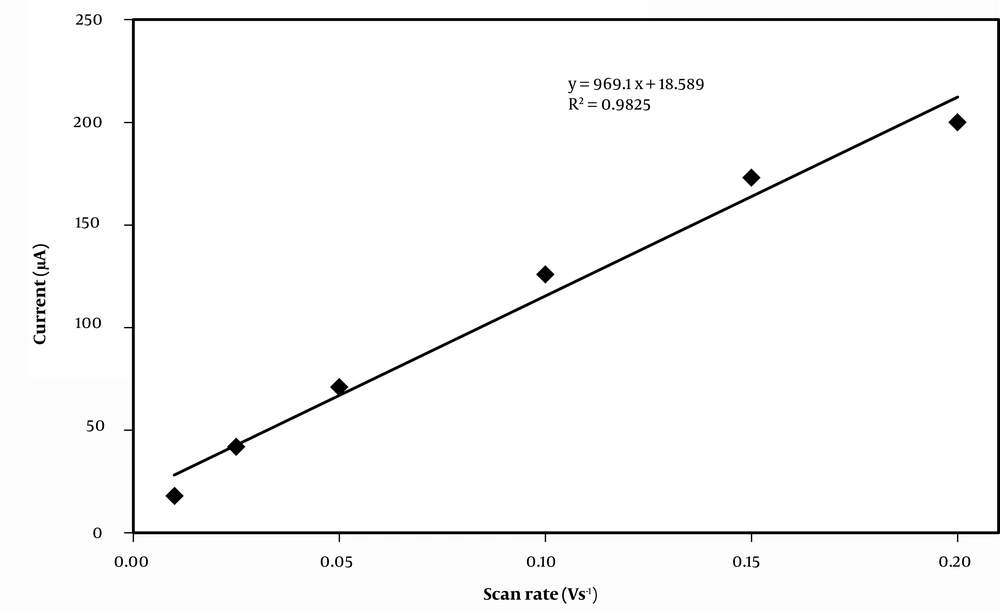
The scan rate expresses rate of the applied potential in cyclic voltammetric experiments. The Randles−Sevcik equation in electrochemically reversible systems shows the linear relationship between anodic current with the square root of the scan rate (Equation 5).
In the above equation ip is the anodic current (A), v is the scan rate (V s-1), n is the number of transferred electrons, A (cm2) is the electrode surface area, Do is the diffusion coefficient of the oxidized species (cm2 s-1), and C0 is the bulk concentration of the analyte (mol cm-3). For reversible processes, it is expected that current to change linearly with increasing scan rates (42). Therefore, the Randles-Sevcik equation and plots of ip versus v1/2 shows whether an analyte is freely diffusing in solution. The effect of different scan rates on oxidation of ethanol onto carbon cloth/PMG/PAMAM/MWCNTs/ADH was investigated by cyclic voltammetry (Figure 5). As the Figure 6 shows, anodic peak current is increased linearly with increasing the scan rate from 0.001 to 0.2 V s-1.
3.4. Conclusions
In this study, we used PMG, PAMAM dendrimer and MWCNTs with layer by layer method to do the immobilization of the ADH. The immobilization of MWCNTs on PAMAM film causes facilitate the connection between enzyme and transducer materials, better enzyme orientation and higher enzyme loading. PAMAM dendrimer due to its porous and organizational structure facilitates the transfer of reduced coenzyme to the electrode surface. Therefore, it plays an important role in the reconstruction of the immobilized ADH cycle. Also, the reaction of ADH and PMG has been studied in homogeneous system using cyclic voltammetry and Albery's analytical equations. It is suggested that the combination of simulation and experiment is a more accurate method to study kinetic parameters of enzyme/substrate/mediator systems. However According to the obtained results, MWCNTs and PAMAM create a convenient environment for effective electronic communication between the center redox of enzyme and the electrode surface.