1. Context
The “Cardiorenal Syndrome “term for the first time was coined by Robert Bright in 1836 following the discovery of the fact that patients with kidney dysfunction and urea secretion in urine showed evidence of cardiovascular problems at the same time (1). Since then, despite many studies regarding the relationship between these two organs, it was the first time in 2008 that the “Acute Dialysis Quality Initiative” introduced two main groups (cardiorenal and reno-cardiac, based on the initial pathology), for this syndrome, and after that, new divisions up to five groups were defined (2). Although this subject is very challenging, because due to close cross-talk of related signaling pathways between these two organs, many times it is not exactly clear whether the initial onset of the defect was from the kidney or from the heart. In general, this term is used in the cases with dysfunction of kidney, heart and finally both of them, which with its progress and lack of proper treatment, leads to multi-organ failure (3).
At first, failure in heart pumping, as a result of volume retention by the kidneys, was known as the main mechanism of this syndrome, but later evidence showed that various items are participated in the formation of this pathogenic puzzle including over-activity of renal sympathetic nervous system, endotoxemia, inflammatory processes, metabolic derangements, infections, imbalance in neurohormones secretion, venous congestion and immunological dysfunction (4, 5). Unfortunately, this syndrome imposes major problems on patients and the medical community and is globally associated with high morbidity and mortality.
Also, specific metabolic changes occur in response to this syndrome, including: Uremia, metabolic acidosis, renovascular reactivity failure, decreased aorta responsivity, heart and kidney failure, increased blood level of C-reactive protein and inflammatory cytokines, and also transient activation of renin–angiotensin system (increasing angiotensin II receptor type 1(AT1) and angiotensin II receptor type 2 (AT2)) (6-8).
The treatment of cardiorenal syndrome involves addressing the underlying cause and managing both cardiac and renal dysfunction. One potential therapeutic approach is renal denervation, which plays a crucial role in managing this condition. Renal denervation is a minimally invasive procedure that involves using radiofrequency energy to disrupt the nerves surrounding the renal arteries. These nerves play a significant role in regulating blood pressure and fluid balance. By interrupting their activity, renal denervation can help reduce sympathetic nerve overactivity, which is often seen in patients with cardiorenal syndrome. This reduction in sympathetic tone leads to improved blood pressure control and decreased fluid retention, ultimately alleviating the strain on both the heart and kidneys. Renal denervation has shown promising results in improving cardiac and renal function in patients with cardiorenal syndrome. Studies have demonstrated that this procedure can lead to a reduction in blood pressure, improved left ventricular function, and a decrease in proteinuria.
Considering above information, this article review aims to collect available data related to cardiorenal syndrome and its therapeutic strategy, mainly by focusing on “renal denervation” as an effective therapeutic approach.
2. Clinical Diagnosis Value of Micrornas and Long Non-coding RNAs in Kidney Heart Diseases
Despite transcripting a large part of the mammalian genome into RNA, only a few of these products are translated into proteins and it is interesting that these non-coding RNAs (ncRNAs) importantly take part in the regulation of RNAs activity, protein function, cellular physiology and disease progression (9). Although, despite the increase of human science about these ncRNAs, the exact number of them and all their functions are still not known, but many diagnostic ncRNAs, especially microRNAs, in specific diseases such as cardio-renal diseases have been known so far (10). Identifying the role of these microRNAs, as diagnostic biomarkers, and the changes in their expression levels in disease conditions can open a new horizon in diagnosing diseases and subsequently finding new treatment solutions. Dysregulated miRNAs have been shown to contribute to cardiac and renal dysfunction in Cardiorenal Syndrome (CRS) by targeting genes involved in processes such as inflammation, fibrosis, and apoptosis. For example, miR-21, which is upregulated in both cardiac and renal tissues in CRS, promotes fibrosis by targeting anti-fibrotic genes. In addition, it has been proven that miR-1, miR-133, miR-26, miR-29, and miR-21 are key players of ischemic heart disease and affect arrhythmia, cell death, hypertrophy, and fibrosis in patients (11). Also, some long non-coding RNAs (lncRNAs) are known as diagnostic biomarkers of kidney dysfunction, like TUG1 for diabetic glomerulopathy (12).
In the context of cardiorenal syndrome, ncRNAs have been found to be involved in various biological processes that contribute to the development and progression of the disease.
Recent studies have identified several lncRNAs that are dysregulated in CRS and contribute to the disease's pathogenesis. These lncRNAs can act as molecular sponges for miRNAs, regulating their availability and activity. Additionally, lncRNAs can interact with chromatin and modulate gene expression. For example, the lncRNA MALAT1 is upregulated in CRS and promotes cardiac fibrosis by interacting with chromatin and activating pro-fibrotic genes.
Studies have shown that kidney denervation, a procedure that involves the ablation of renal sympathetic nerves, can lead to changes in the expression of certain miRNAs. For example, it has been observed that kidney denervation can upregulate miR-132, which is known to be involved in the regulation of blood pressure. This suggests that miRNAs may play a role in the physiological response to kidney denervation and could potentially be used as biomarkers or therapeutic targets for monitoring or modulating the effects of this procedure.
Furthermore, miRNAs have also been implicated in the development and progression of kidney diseases, such as hypertension-induced renal injury and diabetic nephropathy. These conditions often involve dysregulation of the renin-angiotensin-aldosterone system (RAAS), which is targeted by kidney denervation. Therefore, understanding the role of miRNAs in these diseases and their potential modulation by kidney denervation could provide insights into the mechanisms underlying the therapeutic effects of this procedure and help identify new therapeutic strategies for managing kidney diseases.
3. Renal Denervation as a Therapeutic Approach for Cardiorenal Syndrome
Renal denervation (RDN) is a minimally invasive procedure to deal with cardiorenal syndrome, which was first performed in 1924 by Papin & Ambard (13). This procedure is done by burning the nerves in the renal arteries with radiofrequency ablation (14).
One of the golden key players in the scenario of Cardiorenal Syndrome is the elevation of sympathetic vasomotor activity which has made this nervous system as one of the main therapeutic targets of this syndrome (15). Evidence shows that the sympathetic nerve system has a critical role in regulating body homeostasis, especially controlling body fluids and blood pressure (16, 17). On the other hand, it has been proven that dysfunction of this system causes cardiorenal diseases, including heart failure (HF), chronic kidney disease (CKD) and hypertension (18, 19). It has also been determined that renal sympathetic nerves are the key players of the pathogenesis and progression of the mentioned diseases (20).
Regarding this information and the obtained results of many human and animal studies (21-24), renal denervation is known to be a useful therapeutic intervention in the direction of reducing the activity of the sympathetic nervous system (25). The emergence of the idea that the renal nerves affect the kidney function and subsequently the cardiovascular function, goes back to Claude Bernard research in 1859 which showed that diuresis occurs after cutting the greater splanchnic nerve (renal denervation), and antidiuresis occurs after the electrical stimulation of these nerves (26). Later, more studies also showed that the renal denervation is associated with the elevation of renal blood flow (RBF) and the stimulation of these nerves is related to the decrease of RBF (27, 28). Finally, the result of all this research led to the discovery of the fact that renal denervation affects many diseases belongs to cardiorenal syndrome, including blood pressure and heart and/or kidney problems.
4. Renal Denervation in Animal Studies
Until today, many animal studies have been performed in the field of renal denervation and its effects on cardiorenal syndrome which have examined the safety and effectiveness of this treatment method. Table 1 summarizes some of this information.
| Animal Model | CRS-Induction Method | Sample Size | Target Problems | Main Outcomes | Ref |
|---|---|---|---|---|---|
| Sprague-Dawley rats | AMI-model (induction by IP injection of pentobarbital, solution (0.3 ml/100 g) | 32 male rats | Ventricular arrhythmia | RD reduced the occurrence of ventricular tachycardia in AMI rats, RSN discharge and inhibit the activity of local SN. | (29) |
| LQTs-rabbit models | Infusion of infusion of HMR-1556, erythromycin and veratridine respectively for induction of LQT1, LQT2 and LQT3 | 44 | Ventricular arrhythmia | RD significantly reduced the ventricular arrhythmia inducibility in rabbits. | (30) |
| Cardiomyopathy- induced Sprague Dawley rats | Cardiomyopathy-model (induction by IP injection of isoproterenol, 5 mg/kg/d) | 60 male rats (control: 10, Intervention :50) | Cardiomyopathy | RDN inhibits cardio-renal fibrogenesis by reducing SNS over-activity and rebalancing RAAS axis. | (31) |
| 2-kidney, 1-clip (2K-1C) rat model | Clip implantation | NM | Hypertension | Sympathetic overactivity, brain oxidative stress, and renal injury was reduced by RDN. | (32) |
| Sprague-Dawley rats | Salty-dietary regimen was used for normal rats feeding. | 11 | Arterial pressure | RD significantly reduced the arterial pressure in normal rats consuming salty-dietary regimen. | (33) |
| Rat Model of Anti–Thy-1.1 Nephritis | Glomerulonephritis (induction by injecting the monoclonal anti–Thy-1.1 antibody OX-7) | NM | Glomerulonephritis | Glomerulonephritis and albuminuria significantly reduced by RD. | (34) |
| mongrel dogs | AMI-model (induction by specific surgical procedure, briefly by punctuation of right femoral artery) | 18 (8 male and 10 female) | Acute myocardial, infarction (MI) | RDN showed a protective effect against acute MI and decreased the local activity of the SNS and RAS. | (35) |
| Dahl salt-sensitive hypertensive rats | Glomerular injury induced by uninephrectomy (removed right kidney) | 20 | Glomerular injury | RDN reduced ROS in glomeruli and improved renal damage. | (36) |
A Summary of Renal Denervation' Effects on Cardiorenal Syndrome in Some Animal Studies
As shown in Table 1, renal denervation with different mechanisms has been very useful in overcoming the problems of cardiorenal syndrome in animal models and has led to the improvement of their conditions. These brilliant results are promising and have led to the use of this method in human clinical trials.
5. Renal Denervation in Clinical Trial Studies
Using the renal denervation to combat cardiorenal syndrome' problems is known as a new treatment strategy today. In this method, radiofrequency energy, ultrasound waves, or biochemical substances are used to destroy the renal nerves in the wall of the renal artery, and as a result, reduce the sympathetic signals entering and leaving the kidney (37, 38). Although the safety and effectiveness of this method has been promising in many clinical trials (39-41), there are still contradictions in this field (42-44). Table 2 summarizes some clinical trials in this field.
| Type of Study | Sample Size | RD Method | Target Disease | Follow Up Period | Main Outcomes | Ref |
|---|---|---|---|---|---|---|
| Multicenter, randomized trial | 106 | Catheter-based renal denervation | Resistant hypertension | 12 months | A significant reduction of blood pressure was observed. | (45) |
| Clinical trial | 153 | radiofrequency ablation | Resistant hypertension | 36 | A significant decline in blood pressure was reported. | (46) |
| Randomized- sham-controlled trial | 535 (RD: Shame =2:1) | Radiofrequency energy delivered by the Symplicit renal-denervation Catheter (Medtronic). | Severe resistant hypertension | 6 months | An in-significant decline in systolic blood pressure was observed. | (44) |
| Multicenter, randomized- sham-controlled trial | 136 | ultrasound renal denervation | Resistant hypertension | 6 months | A sustained-lower blood pressure during the follow-up period in was observed. | (47) |
| Single-center pilot trial | 8 | catheter-based renal nerve ablation | CKD and uncontrolled hypertension | 6 months | RD reduced blood pressure but had no effect on renal function. | (48) |
| Prospective, open-label, single-arm cohort study | 2237 | RDN catheter insertion | Uncontrolled hypertension and/or conditions associated with sympathetic nervous system activation | 6 months (report from 36 months ongoing follow-up period) | Significant BP reduction and eGFR reduction to the expected range. | (49) |
| Multicenter, randomized- sham-controlled trial | RD:38 Control:42 | Catheter-based renal denervation | Resistant hypertension | 6 months | A significant decrease in blood pressure, with non-severe side effects was reported. | (50) |
| Multicenter, randomized- sham-controlled trial | 133 (RD:1666, Control: 165) | Catheter-based renal denervation | Resistant hypertension | 3 months | A significant decrease in blood pressure, with nom sever side effects, in intervention group compared to control group was observed. | (51) |
| Clinical trial | 46 | Catheter-based renal denervation | Ckd | Up to 24 month | RD improved and stabilized eGFR for up to 24 month in patients. | (52) |
| Cohort clinical trial | 27 | Catheter-based renal denervation using the Symplicity Flex RDN System | CKD and resistant hypertension | Up to 36 month | RDN reduced BP and slowed the decline of renal function. | (53) |
| Randomized Sham-Controlled Trial | 71 (RD: Shame =1:1) | Catheter-based renal denervation using the Symplicity Flex RDN System | Mild resistant hypertension | 6 months | RDN reduced BP and was safe and well tolerated. | (54) |
| Pilot clinical trial | 15 (9 men & 6 women) | Catheter-based renal denervation | Resistant hypertension (grade 3) and CKD Stage 3 - 4 | 12 months | The mean reduction in office blood pressure, significantly decrease in night-time ambulatory blood pressure, and preserved renal function was reported. | (55) |
| Clinical trial | 24 (9 men and 15 women) | Radiofrequency energy delivered by the symplicity renal-denervation Catheter | CKD (stage 2,3,4) and resistant hypertension | 6 months | An improved BP control and a short-term raise in eGFR was reported. | (56) |
| Randomized- controlled trial | 100 (RD: 88, Control: 12) | Ultrasound renal denervation | Resistant hypertension | 6 months | RD reduced BP, renal resistive index, and incidence of albuminuria without adversely affecting glomerular filtration rate or renal artery structure. | (57) |
A Summary of Renal Denervation' Effects on Cardiorenal Syndrome in Some Clinical trials
6. Renal Denervation Disadvantage
Renal denervation is a procedure that involves the ablation of renal nerves in order to treat conditions such as hypertension. While it has shown promising results in some patients, there are several disadvantages and limitations associated with this procedure.
One of the main disadvantages of renal denervation is the lack of consistent and long-term efficacy. While initial studies showed significant reductions in blood pressure following renal denervation, subsequent trials have yielded mixed results. Some patients experience a sustained reduction in blood pressure, while others show no significant improvement. The reasons for this variability in response are not fully understood, but it is believed to be influenced by factors such as patient characteristics, procedural technique, and the presence of secondary causes of hypertension. This lack of consistent efficacy limits the widespread adoption of renal denervation as a standard treatment for hypertension.
Another disadvantage of renal denervation is the potential for procedural complications. Although the procedure is minimally invasive, there are risks associated with it. These include renal artery dissection or perforation, renal artery stenosis, renal infarction, and access site complications. While these complications are relatively rare, they can have serious consequences for patients. Additionally, the long-term effects of renal denervation on renal function are not well understood, and there is a concern that it may lead to renal artery stenosis or ischemia in some cases.
Furthermore, renal denervation is not suitable for all patients with hypertension. The procedure is typically reserved for patients with resistant hypertension who have failed to achieve adequate blood pressure control despite optimal medical therapy. However, not all patients with resistant hypertension will benefit from renal denervation. It is estimated that only a small proportion of patients with resistant hypertension have a truly sympathetic-driven form of the condition that can be effectively treated with renal denervation. Therefore, careful patient selection is crucial to ensure that the procedure is performed in those who are most likely to benefit.
Additionally, the optimal technique for renal denervation has not been established, and there is variability in procedural approaches among different centers. This lack of standardization makes it difficult to compare results across studies and limits the ability to draw definitive conclusions about the effectiveness of renal denervation.
7. Renal Sympathetic Nerves and its Interaction with the Renin-Angiotensin System
How does the renin-angiotensin system affect kidney function and cardiorenal syndrome? To find the answer of this question, we must look for the interaction effects of this system with the renal sympathetic nervous system. As studies show, the over-activity of the renal sympathetic nervous system affect the functions of the nephron, the vasculature, and the renin-containing juxtaglomerular granular cells. The overacting of the renin angiotensin system also exerts exactly the same effects on kidney activity. So, it is really crucial to evaluate the interactions between these two systems in controlling renal function (58).
The renin-angiotensin system (RAS) is the main regulator of blood pressure, renal function, and homeostasis of body fluids (59). It is necessary to adjust this system in patients hospitalized in the intensive care unit (ICU) and it is directly accompanied by the changes in clinical conditions of the patients (60, 61). Renin-angiotensin systems are cascade systems in which, with the help of angiotensin-converting enzymes (ACEs), angiotensin is produced from the stepwise breakdown of peptides. As stated by many studies, the renin-angiotensin system consists of several various components and two main axis of signaling pathways named classical and non-classical axis (62, 63). In a classical pathway, angiotensin (Ang) II produced from Ang I with the help of angiotensin-converting enzyme (64). In physiological conditions, Ang II binds to its own receptor in the adrenal cortex, causing the release of aldosterone and sodium reabsorption in the kidneys (65). Also, the Ang (1-7)/ACE2 cascade is known as the non-classic RAS (66). It is proven that Ang II, the main effector peptide of RAS, is directly associated with many cases of kidney damages (67). By acting on its two main receptors (AT1R & AT2R) this peptide causes increased cell proliferation, inflammation and fibrotic damage in the kidney parenchyma by up-regulating inflammatory mediators of some specific signaling pathways, including NF-Kβ, interleukin 6, and TNF-α and stimulation of fibroblasts (67, 68).
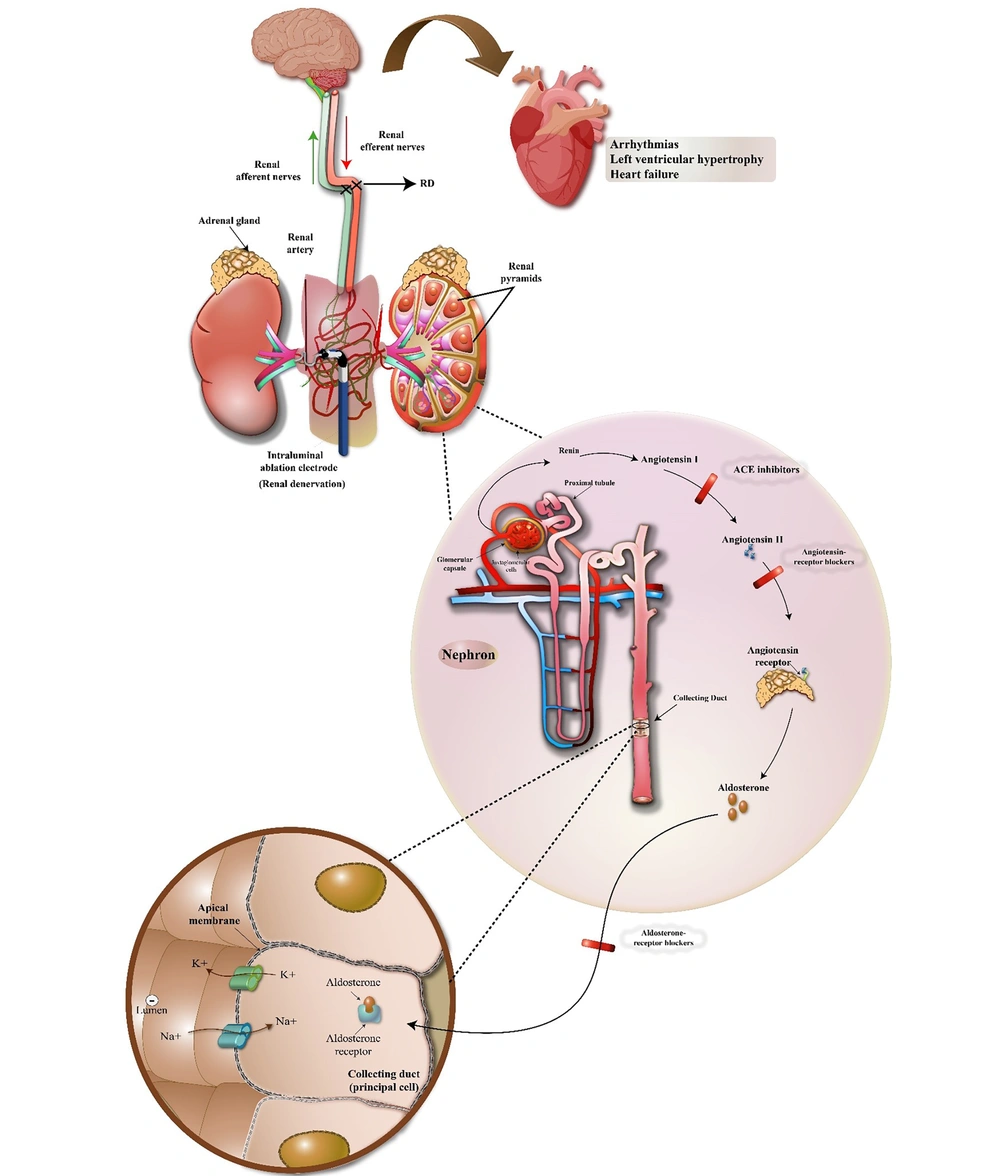
It is well established that increased levels of circulating angiotensin II and angiotensin II originated from central nervous system (CNS) can affect the renal sympathetic nerves activity and subsequently renal function (69, 70). On the other hand, since the increase in the activity of both renal sympathetic nervous system and the renin-angiotensin system has a direct relationship with the progression of kidney-heart diseases, therefore, regulating the excess activity of these systems by renal denervation method is considered a useful therapeutic solution in cardio-renal syndrome (71). There are many studies that show that renal denervation significantly reduces the amount of renal norepinephrine and circulating angiotensin (I & II) and increases cardio-renal function (72, 73). Renal denervation regulates Ang II receptor expression in kidneys and affect renal function (74). In this regard, Figure 1 schematically shows the effects of the renal sympathetic system and its denervation on the renin-angiotensin system and subsequently cardiorenal syndrome.
Excessive activity of the sympathetic nervous system can lead to dysfunction of both the heart and kidney organs, so that it affects the heart and can lead to arrhythmias, left ventricular hypertrophy and heart failure. Also, over activity of this nervous system in the kidneys leads to an increase in the activity of the renin-angiotensin system, and as a result, the juxtaglomerular cells in the kidney, by activating prorenin molecules, secrete renin directly into the blood. Then renin, in turn, converts the angiotensinogen secreted by the liver into angiotensin I. Angiotensin I is converted to angiotensin II by angiotensin-converting enzyme present in the lungs. Angiotensin II is a vasoconstrictor peptide that increases blood pressure by narrowing the arteries. Angiotensin II also stimulates the secretion of aldosterone hormone from the cortical part of the adrenal gland. Aldosterone increases sodium and water absorption from kidney tubules. With more water and sodium absorption and as a result of increasing blood volume, blood pressure increases. If the renin-angiotensin-aldosterone system is abnormally activated, blood pressure increases too much. There are many drugs that reduce blood pressure by inhibiting various stages of this system including Angiotensin Converting Enzyme Inhibitors, Angiotensin Receptor Blockers and aldosterone receptor blockers and as a result, they can be useful in the treatment of cardiorenal syndrome disorders. Also, by preventing the formation of this enzymatic cascade, renal denervation can lead to the improvement of the disorders of this syndrome to a large extent.
8. Current Treatment Strategies for Cardiorenal Syndrome
Today, cardiorenal syndrome is known as a progressive complication among patients with heart failure and kidney disorders. Therefore, access to treatment strategies are very important in this field. In addition to renal denervation, there are other evidence-based treatment strategies, including: Using angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB), loop diuretic and thiazides, dopamine and natriuretic peptides, which are briefly mentioned below. However, new treatment strategies are also emerging (like targeting non-coding microRNAs) that prove their effectiveness is still in the experimental and study stages.
8.1. Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
It was in 2012 that the use of ACEI was recommended by the European Society of Cardiology (ESC) for the treatment of all heart failure-hospitalized patients with an ejection fraction less than 40%, to reduce the risk of premature death. Also, ARB was suggested for patients who could not tolerate the side effects of ACEI (75). So far, many experimental and clinical studies have proven the beneficial therapeutic effects of these drugs in cardio-renal problems. For example, promising results were reported in a 2012 cohort study by Ahmed et al. on 1665 patients, 1046 of whom received these drugs. The obtained results determinded that the use of ACEI and ARB drugs had a significant relationship with the reduction of death factors, especially in elderly patients with systolic heart failure and chronic kidney disease (76). Also, in another large contemporary cohort study on CKD patients, the use of these drugs showed a direct relationship with increased patient survival (77). The common finding of all these studies was a decrease in glomerular filtration to reduce the intraglomerular pressure gradient and a slight increase in serum creatinine levels in patients. Reducing proteinuria is another benefit of these drugs. Although each of ACEIs or ARBs alone is able to reduce proteinuria, but the combination of these two drugs has shown a stronger therapeutic effect in this field (78). The exact mechanism by which ACE inhibitors and ARBs cause a decrease in glomerular filtration and reduce the intraglomerular pressure gradient is as follows:
1. Renin release inhibition: ACE inhibitors and ARBs block the production or action of angiotensin II, a potent vasoconstrictor. This leads to a decrease in the release of renin, an enzyme involved in the conversion of angiotensinogen to angiotensin I.
2. Decreased angiotensin II formation: ACE inhibitors directly inhibit the enzyme ACE, responsible for converting angiotensin I to angiotensin II. ARBs, on the other hand, block the binding of angiotensin II to its receptors. Both actions result in decreased levels of angiotensin II in the body.
3. Vasodilation of efferent arterioles: Angiotensin II is a potent vasoconstrictor that constricts both afferent and efferent arterioles in the kidneys. By inhibiting its production or action, ACE inhibitors and ARBs cause selective vasodilation of the efferent arterioles while minimally affecting the afferent arterioles. This preferential dilation of the efferent arterioles reduces the intraglomerular pressure gradient.
4. Reduced glomerular filtration rate (GFR): The constriction of efferent arterioles by angiotensin II normally helps maintain a higher intraglomerular pressure, promoting filtration of blood through the glomerulus. By inhibiting this vasoconstrictor effect, ACE inhibitors and ARBs decrease the intraglomerular pressure and subsequently reduce the GFR.
8.2. Loop Diuretics
Loop diuretics are a class of drugs that are primarily used to treat conditions such as edema and hypertension. They work by inhibiting the reabsorption of sodium and chloride in the ascending loop of Henle in the kidney, leading to increased urine production and decreased fluid volume. Diuretics have always been common treatments in heart-failure disease to prevent rehospitalization. Although the role of these drugs like loop diuretics and thiazides in reducing the mortality of patients with heart-failure disease has not been proven in general, but their role in accelerating the recovery and reducing the symptoms of the disease has been proven in many studies (79, 80). One of the main indications for loop diuretics is congestive heart failure. In CHF, the heart is unable to pump blood effectively, leading to fluid accumulation in the body, particularly in the lungs and extremities. Loop diuretics help to reduce this fluid overload by increasing urine production and promoting the excretion of excess fluid. By reducing fluid volume, loop diuretics can relieve symptoms such as shortness of breath and swelling in patients with CHF.
Loop diuretics are also commonly used in the management of acute pulmonary edema. This condition occurs when there is a sudden accumulation of fluid in the lungs, typically due to heart failure or other causes. Loop diuretics can help to rapidly remove excess fluid from the body, relieving symptoms and improving oxygenation.
In addition to CHF and acute pulmonary edema, loop diuretics may also be used in the treatment of other conditions such as cirrhosis, nephrotic syndrome, and certain types of hypertension. However, it is important to note that loop diuretics are not suitable for all patients, and their use should be carefully monitored by a healthcare professional.
Of course, it should be very careful that high doses of diuretics have the opposite effect and increase the death rate in patients with heart-failure disease (81).
8.3. Dopamine and Natriuretic Peptides
The first treatment line of all types of cardiorenal syndrome is to focus on maintaining systolic blood pressure by adrenergic agents. Dopamine is a strong stimulator of adrenergic receptors (α and β), with the same vasopressor effects as norepinephrine, but more adverse effects (at low doses), that leads to various biological effects (82). Although studies show that this treatment reduces many causes of hospitalization or death in patients (83), but in order to prevent its unwanted side effects on kidney function (in high doses), it needs to be cautious and conduct more clinical trials.
Dopamine and natriuretic peptides play complex roles in the cardiorenal syndrome. Their effects can vary depending on the specific context and stage of the syndrome. Dopamine is a neurotransmitter and hormone that has various functions in the body, including its role in the cardiovascular system. In the context of cardiorenal syndrome, dopamine can have both beneficial and detrimental effects. It has been used as a therapy to improve renal function in certain situations, such as acute kidney injury or low cardiac output states. Dopamine can help increase renal blood flow and promote diuresis, which can be beneficial in reducing fluid overload and improving kidney function. However, the use of dopamine in cardiorenal syndrome is still a topic of debate and ongoing research. Some studies have shown limited or no benefit from dopamine therapy, and there are concerns about potential side effects, such as arrhythmias or worsening heart function. Therefore, the use of dopamine in cardiorenal syndrome should be carefully considered on a case-by-case basis, with close monitoring by healthcare professionals.
Natriuretic peptides, including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), are hormones produced by the heart in response to increased pressure or stretch. They have important roles in regulating fluid balance and blood pressure. In the context of cardiorenal syndrome, elevated levels of natriuretic peptides are often seen due to heart failure or other cardiac conditions. Natriuretic peptides have beneficial effects on the kidneys by promoting vasodilation of the renal blood vessels and increasing sodium excretion. This can help reduce fluid overload and improve renal function. However, in advanced stages of heart failure or severe cardiorenal syndrome, the ability of natriuretic peptides to improve renal function may be impaired.
Therefore, while natriuretic peptides initially act as beneficial compensatory mechanisms to counteract fluid overload and maintain cardiac output, their long-term effects in cardiorenal syndrome can be more complex. The levels of natriuretic peptides can be influenced by various factors, including renal function, medications, and comorbidities, making their interpretation and clinical management challenging.
9. Discussion
The aim of this review is to investigate cardiorenal syndrome and its treatment strategies, focusing on the renal denervation method and the basic factors involved in it, including the interactions of the renin-angiotensin system with the renal nerves. As mentioned above, Cardiorenal syndrome refers to a pathological condition in which the heart and kidney are damaged at the same time, and eventually, if left untreated, this damage will affect other parts of the body systemically (84). Therefore, finding therapeutic ways to overcome this problem is considered one of the necessities of medical science. So far, many treatment methods have been used for this syndrome, including loop diuretics, dopamine and natriuretic peptides, angiotensin converting enzyme inhibitors and angiotensin receptor blockers, each of which has its advantages and disadvantages (75). With a glance look at the mechanism of this syndrome, it is clear that the role of excessive activation of the sympathetic nervous system and subsequently the renal nerves in the formation of the disease is very prominent. respecting this information, renal denervation via various ways, can definitely be an excellent medical option for this syndrome. So far, many animal and human studies have confirmed the safety and effectiveness of this treatment method (Tables 1 and 2). On the other hand, studies show that excessive activation of the sympathetic nervous system causes stimulation of the renal nervous system activity and over-activation of the renin-angiotensin system. In a positive feedback loop, the over-activation of the renin-angiotensin system subsequently has the same effect on the renal nervous system and its activation (63). And this vicious circle of stimulation, if not inhibited by an intervention, will have a negative effect on the heart and other organs of the body in a short period of time (due to the key role of the renin-angiotensin system in regulating body fluids and blood pressure). There is a lot of evidence that over-activity of the renin-angiotensin system leads to disruption of body fluid balance and blood pressure, increased oxidative stress, renal fibrosis, cell proliferation, and inflammatory processes (85, 86).
Studies also show that angiotensin II, which is produced in the classical pathway of the renin angiotensin system under the influence of enzyme ACE from angiotensin I, plays a key role in causing many kidney problems and subsequently cardiorenal syndrome, and the main reason for the use of angiotensin inhibitor drugs is the same. Renal denervation can also regulates Ang II receptor expression in kidneys and affect renal function (25). Since this method is minimally invasive and is able to regulate the renin-angiotensin system in a shorter time than drug treatments and solve systemic problems including kidney problems, its use in affected patients is considered a promising window in improving the disease.
9.1. Conclusions
Cardiorenal syndrome is a general term for pathological conditions in which heart and kidney dysfunction occur simultaneously. Heart failure, chronic kidney disease and hypertension are considered to be one of the main disorders of this syndrome, which lead to multi-organ failure if not controlled and treated. Therefore, finding effective treatment solutions to overcome the problems of this syndrome is one of the biggest challenges of medical science. Since the over-activity of the renal sympathetic nervous system and the subsequent increase in its interaction with the renin-angiotensin system (both classical and non-classical axes) are considered to be the main causes of the onset of this syndrome, renal denervation is one of the main and effective therapeutic solutions in this field, which its safety and effectiveness have been proven in many experimental and clinical studies so far. Renal denervation can help reduce sympathetic nerve overactivity, which is often seen in patients with cardiorenal syndrome. This reduction in sympathetic tone leads to improved blood pressure control and decreased fluid retention, ultimately alleviating the strain on both the heart and kidneys. Regarding this information, the use of renin angiotensin system blockers has also been effective to a large extent to overcome the problems of this syndrome. Certainly, more clinical studies are needed to find other treatment strategies.