1. Background
Colorectal cancer (CC) is a significant cause of mortality worldwide. The standard treatments for CC significantly contributes to human mortality. Generally, the standard treatments for CC include radiotherapy, chemotherapy, and surgery (1). However, chemotherapy and radiotherapy often cause numerous side effects, including blood abnormalities, constipation, nervous system damage, memory issues, anorexia, and alopecia (2).
Approximately 80% of the global population relies on traditional treatments such as phytotherapy or phytomedicine. Given the well-documented positive effects of medicinal plants, many studies suggest their potential benefits in cancer prevention. Various bioactive molecules have been identified in different parts of medicinal plants (3). Diosgenin, a steroidal sapogenin found in the seeds of Trigonella foenum-graecum, has demonstrated multiple biological activities, including anti-neoplastic and pro-apoptotic effects across various human cancers. The molecular mechanism of diosgenin is linked to its ability to modulate multiple cell signaling pathways, influencing key cellular processes (4).
Pin1 directly interacts with several signaling pathways, including Wnt (5), which plays a pivotal role in colon tumorigenesis (5-9). β-catenin, a key downstream effector in the Wnt signaling cascade, is implicated in the progression of human colon cancer. Its nuclear translocation is a crucial event in the activation of this oncogenic pathway, as demonstrated in previous research (10). Cyclin D1, a β-catenin-regulated gene, is identified as a critical target of the Wnt signaling pathway (11, 12). Its nuclear expression is significantly elevated in colon cancer cases, suggesting its involvement in tumorigenesis (13). Studies indicate that in more than 80% of CC cases, the APC gene is mutated (14, 15), triggering the activation of oncogenes c-myc and cyclin D1 (16, 17).
The serine/threonine-proline (Ser/Thr-Pro) motif is a crucial phosphorylation site for various proline-directed kinases (18). Pin1 selectively catalyzes the isomerization of the Ser246/Thr-Pro motif in the β-catenin protein, enhancing its stability (19). Pin1's role in β-catenin stabilization in CC is mediated by its binding to the β-catenin-APC complex, inhibiting β-catenin's binding to APC, leading to its accumulation in the nucleus. Pin1 also stabilizes cyclin D1 by phosphorylating its Thr286-Pro site, further stabilizing the protein (20). In a study conducted on MCF-7 breast cancer cells, Pin1 was found to play a crucial role in signaling pathways involving cyclin D and β-catenin (19). Immunohistochemical analysis of CC samples revealed significantly elevated β-catenin, cyclin D1, and Pin1 (βCP) levels, highlighting the critical role of Pin1 in β-catenin activity (21).
2. Objectives
Given the crucial role of the Wnt/β-catenin signaling pathway in CC development and the notable anticancer properties of Diosgenin, this laboratory study aimed to evaluate the effects of Diosgenin on the gene expression of key components in the Wnt/β-catenin signaling pathway in CC cells.
3. Methods
3.1. Cell Culture
The SW 742 CC cell line (Pasteur Institute, Iran) was cultured in RPMI-1640 medium (Gibco, Germany) containing 10% inactivated fetal bovine serum (FBS) (Gibco, Germany) and 2 mM glutamine (Gibco, Germany) (22).
3.2. MTT Assay
A total of 15,000 CC cells were seeded and incubated overnight. The cells were then treated with various concentrations of Diosgenin (Merck, Germany) (1, 2, 4, 8, 16, 32, 64, and 128 µM). Control cells received culture medium without the treatment drug. After 24, 48, 72, and 96 hours, MTT solution (5mg/mL) (Merck, Germany) was added and incubated for 3 hours in a dark environment. Following incubation, the solution in each well was drained, and 200 µL of DMSO (Merck, Germany) was added to the wells. After 20 minutes, the absorbance was measured at 570 nm. The cell viability rate (%) was calculated based on the associated citation (23).
3.3. Cytotoxicity Assay
Colorectal cancer cells (70,000 cells/well) were cultured and incubated overnight at 37°C. Following incubation, the cells were treated and further incubated for 24, 48, and 72 hours. Afterward, 100μL of the conditioned medium from each well was transferred to new wells. Lactate dehydrogenase (LDH) activity was assessed using the Cytotoxicity Detection Kit (Roche Chemical Co., Basel, Switzerland). The optical density (OD) value was measured at 490 nm (24).
3.4. Apoptosis Assay
Colorectal cancer cells were treated with the IC50 concentration of Diosgenin and incubated for 24 hours. Following incubation, the cells were stained using Annexin V-FITC (Abcam Inc., Cambridge, MA, USA) and Propidium Iodide (PI) (Sigma-Aldrich, USA). The stained cells were then analyzed using DML software. DNA fragmentation was assessed using a spectrophotometer at 600 nm (25).
3.5. Gene Expression Assay
Various gene expressions were determined using quantitative PCR (qPCR). Total RNA was extracted using Thermo Fisher Scientific TRIzol reagent (Waltham, MA, USA). Following the quality assessment of the purified RNA, complementary DNA (cDNA) was synthesized using a Vivantis Technologies kit (Selangor DE, Malaysia). β-actin was used as the internal control. Real-time PCR was performed using Takara Bio Inc. SYBR Premix Ex Taq technology (Otsu, Shiga, Japan) on the applied biosystems stepone real-time PCR system (Foster City, CA, USA). The primer sequences were as follows: β-actin (F: CATGTACGTTGCTATCCAGGC, R: CTCCTTAATGTCACGCACGAT) (26), Pin1 (F: TGATCAACGGCTACATCCAG, R: CAAACGAGGCGTCTTCAAAT) (27), β-catenin (F: CATCTACACAGTTTGATGCTGCT, R: GCAGTTTTGTCAGTTCAGGGA) (28), and Cyclin D1 (F: TCTTGGTTACAGTAGCGTAG, R: GTAGCGTATCGTAGGAGTG) (28). Fold change was calculated using the 2-∆∆Ct method (29). For PCR amplification, the following steps were undertaken: An initial step at 55°C for 15 minutes, 93°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 45 seconds.
3.6. Statistical Analysis
Experiments were performed in triplicate and repeated three times. Statistical data were presented as means ± standard error. One-way analysis of variance (ANOVA) with Tukey's post-hoc test was applied to assess the differences between groups. SPSS software (v.16) was used for all statistical data analyses, and a P-value of < 0.05 was considered the significant threshold (30).
4. Results
4.1. The Effects of Diosgenin on Colorectal Cancer Cell Viability
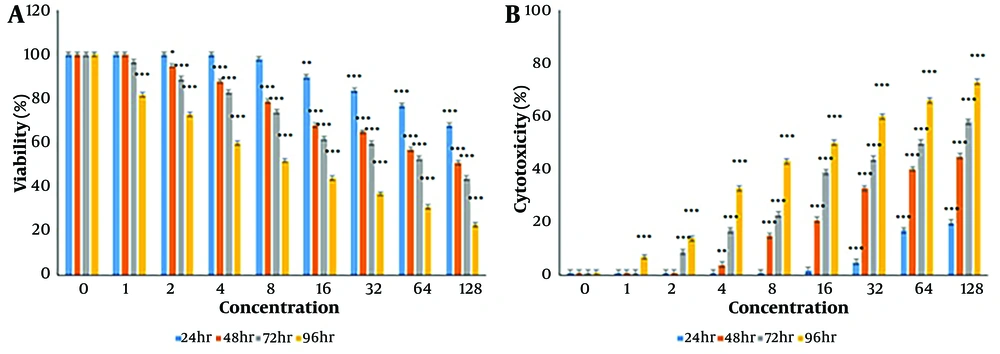
As shown in Figure 1A, Diosgenin treatment significantly reduced cell viability in CC cells in a concentration- and time-dependent manner. The reduction in cell viability was observed at concentrations of 16, 32, 64, and 128 µM after 48 and 72 hours and at concentrations of 2, 4, 8, 16, 32, 64, and 128 μM after 96 hours (P < 0.05). The IC50 values were determined as 203.55 µM, 122.95 µM, 70.11 µM, and 7.34 µM for 24, 48, 72, and 96 hours, respectively.
4.2. The Effects of Diosgenin on Plasma Membrane Integrity in Colorectal Cancer Cells
As illustrated in Figure 1B, Diosgenin demonstrated concentration- and time-dependent cytotoxic effects on CC cells. Significant toxicity was observed after 24 hours at concentrations of 32, 64, and 128 μg/mL, after 48 hours at concentrations of 4, 8, 16, 32, 64, and 128 μg/mL, and at all concentrations after 96 hours.
4.3. The Effects of Diosgenin on Colorectal Cancer Cell Apoptosis
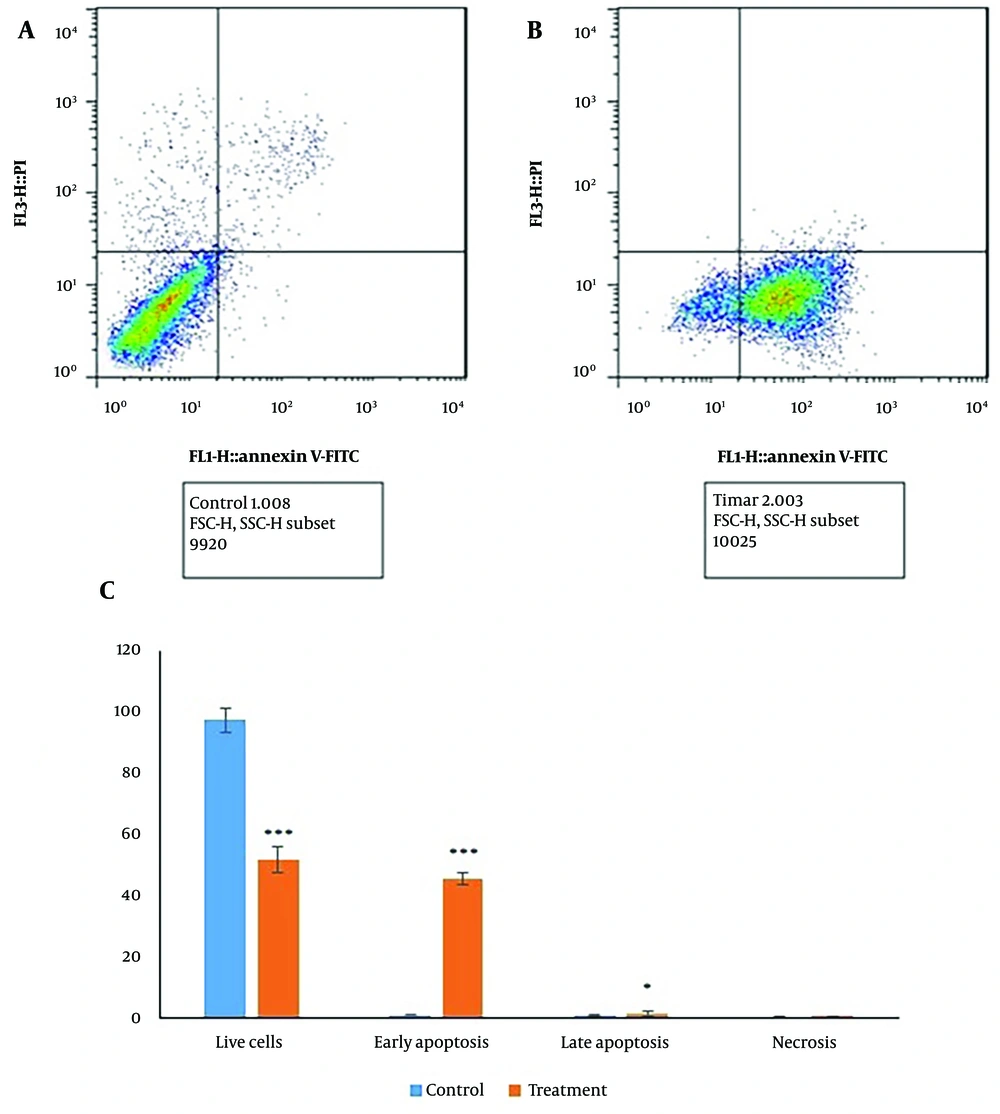
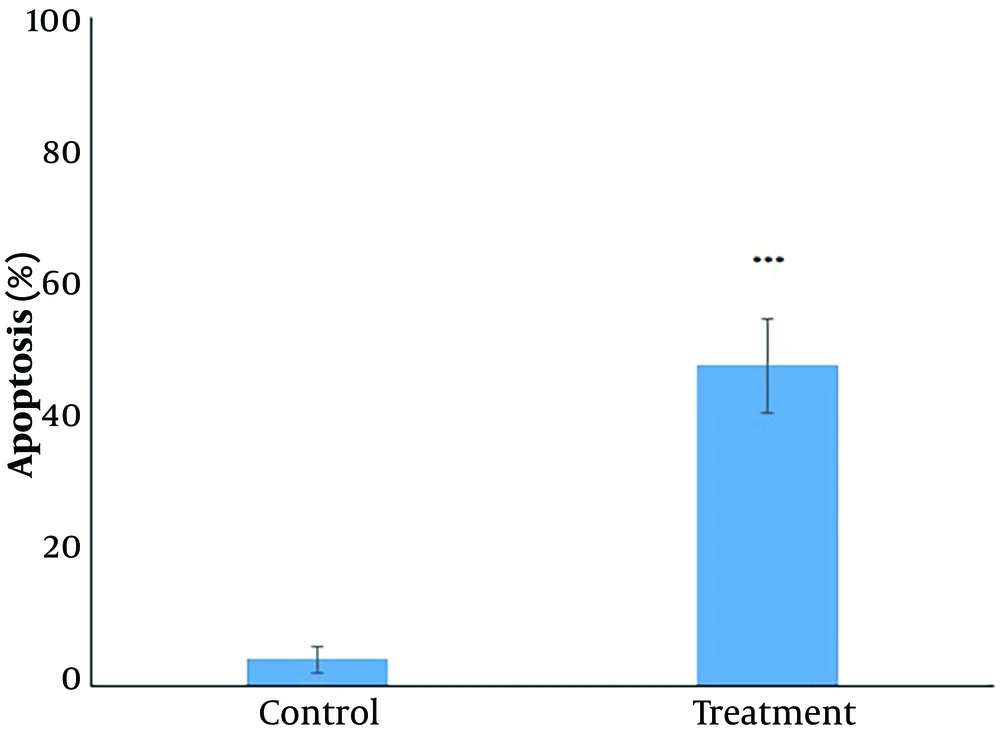
The apoptosis assay results revealed that in untreated CC cells, 97.49% of cells were viable, 0.98% were early apoptotic, and 1% were late apoptotic. In contrast, Diosgenin-treated cells exhibited a significant increase in apoptosis, with 45.64% of cells in early apoptosis and 1.66% in late apoptosis, resulting in a total apoptotic percentage of 47.30% (Figure 2). The Diphenylamine test confirmed a significant (P < 0.05) increase in apoptosis after 24 hours of treatment (Figure 3).
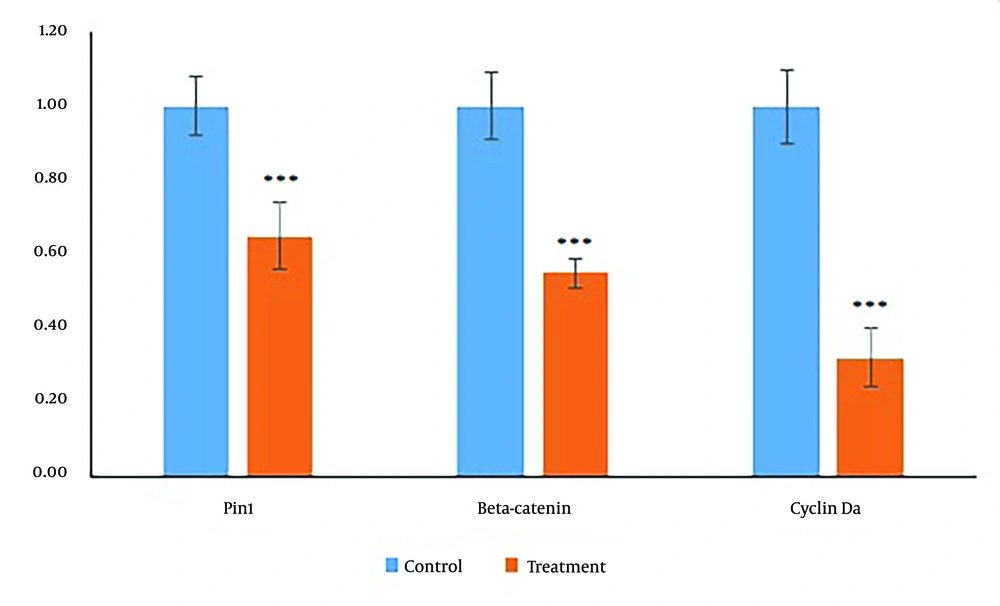
4.5. The Effects of Diosgenin on Gene Expression in Colorectal Cancer Cells
As shown in Figure 4, Diosgenin treatment at its IC50 concentration led to a significant reduction in the expression of β-catenin, cyclin D1, and Pin1 genes (collectively referred to as βCP) after 24 hours (P < 0.05).
5. Discussion
The bioactive compounds derived from medicinal plants are characterized by their low toxicity and multi-targeting properties, which enable them to effectively disrupt oncogenic processes. These plants offer a unique source of enhanced treatment options. The present study found that Diosgenin, a bioactive compound, can potentially decrease CC cell viability in a time- and concentration-dependent manner. The IC50 values for 24, 48, 72, and 96 hours were 203.55, 122.95, 70.11, and 7.34 μM, respectively. Diosgenin has been shown to exhibit concentration- and time-dependent toxicity and induce apoptosis in cancer cells. Previous studies have demonstrated that Diosgenin inhibits cancer growth (31-33) through the modulation of signaling pathways that regulate the cell cycle, differentiation, and apoptosis (34). Diosgenin has also been found to suppress pro-inflammatory genes and activate apoptosis in cancer cells (35). Shishodia and Aggarwal stated that Diosgenin inhibits TNF-α-induced NF-κB activation and suppresses osteoclastogenesis in macrophages (35). Additionally, Diosgenin inhibits AKT and mTOR expression (31). Diosgenin activates the STAT3 signaling pathway in hepatocellular carcinoma and suppresses the transcriptional activity of STAT3 (32). Diosgenin has been shown to inhibit cell proliferation, AKT, and JNK signaling pathways in a concentration- and time-dependent manner, ultimately leading to the induction of apoptosis (36). In myeloid leukemia cells, the autophagic response triggered by Diosgenin effectively blocks the mTOR signaling cascade and induces programmed cell death through apoptosis (37). This specialist also induces cytotoxicity and inhibits the growth and proliferation of breast cancer cells. It has further been demonstrated that Diosgenin inhibits breast cancer induced by the use of N-nitroso-N-methylurea (38). According to studies, Diosgenin is a potent agent for inhibiting tumor growth (38-43). A comprehensive review of the literature on Diosgenin's anticancer properties revealed a lack of studies examining the involvement of the Wnt/β-catenin signaling pathway in its molecular mechanism. However, our research demonstrated that Diosgenin significantly downregulates the expression of βCP genes in CC cells. Moreover, the post-translational accumulation of β-catenin through APC mutations is a key stage in CC (10). A recent study revealed that Pin1 was overexpressed in more than 50% of human hepatocellular carcinoma cases. Additionally, all control groups exhibited concomitant overexpression of Pin1 and β-catenin, with 68% of cases showing simultaneous accumulation of β-catenin and cyclin D1. These findings suggest that Pin1 plays a critical role in the accumulation of β-catenin, a key process in hepatocellular carcinogenesis. Consequently, Pin1 has been identified as a promising therapeutic target for tumor treatment (44). Another study was conducted to elucidate the role of Pin1 in the overexpression of cyclin D1 and β-catenin in follicular adenoma and papillary thyroid cancer. The results indicated that the overexpression of cyclin D1 and the aberrant expression of β-catenin are critical events in the development of thyroid-associated tumors. Notably, Pin1 expression was found to be closely correlated with cyclin D1 levels, highlighting Pin1's significant effect on regulating the expression of cyclin D1 and β-catenin (45). In addition, the expression of Pin1 mRNA and protein was examined in oral cancer cell lines, and the expression of βCP was analyzed in clinical samples using immunohistochemical staining. The study revealed that Pin1 is overexpressed in oral cancer and that its levels are positively correlated with the intracellular levels of cyclin D1. These findings collectively suggest that Pin1 plays an oncogenic role in oral cancers (46). In a comprehensive immunohistochemical analysis of cervical cancer samples, the correlations between Pin1 and β-catenin or cyclin D1 were examined. The study revealed that Pin1 was overexpressed in approximately 40% of CC cases. Furthermore, high Pin1 expression is directly related to β-catenin and cyclin D1 overexpression. These findings suggest that Pin1 has a crucial role in CC progression by accelerating the activity of β-catenin and cyclin D1, key proteins involved in the Wnt/β-catenin and cell cycle pathways, respectively (21). Numerous studies have documented the expression of Pin1 in human cancers, highlighting its potential role in tumorigenesis. Notably, Pin1 overexpression was found in cervical cancer cell lines. In contrast, less than 10% of normal CC cells exhibited Pin1 overexpression. Published research consistently shows that Pin1 expression is positively correlated with histopathological grades of CCs. Significant differences were found between tumors in the submucosa and those beyond the muscular layer. Additionally, Pin1 expression was significantly higher in late tumor stages compared to early stages. These findings collectively support the notion that Pin1 promotes cancer progression by contributing to the development and growth of tumors (18). The expression level of Pin1 does not appear to be associated with the frequency of lymph node metastasis in cancer patients, suggesting that Pin1 may not be essential for tumor cells to acquire metastatic capabilities. Adenomatous polyposis coli (APC) and β-catenin are crucial mediators of the Wnt signaling pathway. Interestingly, while APC mutations are commonly observed in cancer cases, the rate of β-catenin mutations is relatively low in cervical cancer samples. These findings indicate that the dysregulation of the Wnt pathway in CC is more likely driven by APC alterations rather than direct mutations in β-catenin (47). Pin1 initiates β-catenin activity in the nucleus and disrupts the APC-β-catenin complex in breast cancer cells (19). Notably, APC mutations typically occur early in the development of colon cancer, whereas β-catenin expression levels tend to increase during the later stages of tumorigenesis (48, 49). Given the potential role of Pin1 in tumor progression, it is plausible that Pin1 facilitates the accumulation of β-catenin in the late stages of cervical cancer. Furthermore, there is a correlation between β-catenin and cyclin D1 expression in CC cases, suggesting that these proteins may cooperate in promoting tumorigenesis (50). In numerous instances, the elevated expression of Pin1 has been accompanied by increased levels of β-catenin and cyclin D1. Notably, Pin1 has been shown to directly isomerize and stabilize cyclin D1, highlighting its potential role in regulating cell cycle progression. Additionally, studies have demonstrated that prostate cancer patients with high Pin1 expression exhibit poorer survival outcomes compared to those with low or no Pin1 expression, as reported by Ayala et al. (51). These findings collectively support the notion that Pin1 serves as a reliable prognostic marker for prostate cancer, with high expression levels indicating a poor prognosis.
5.1. Conclusions
In light of the key roles of the Wnt/β-catenin signaling pathway in CC development, our study concludes that Diosgenin may exert anti-CC effects by inhibiting the expression of βCP genes. These findings suggest Diosgenin as a promising therapeutic agent for targeting the Wnt/β-catenin pathway in CC.