1. Background
Food contamination may emerge at different stages of preparation, transportation, and supply through unnecessary hand exposures, raw materials (such as milk), or contaminated equipment. Developing countries, given their cultural and economic constraints, face a wide range of food-borne diseases. Also, in developed countries, despite technological advances and use of advanced methods, 5% - 10% of people are affected by food-borne diseases, causing health and economic problems in communities (1).
According to the World Health Organization (WHO), food-borne diseases are described as infectious diseases, induced by the consumption of water or food. Food poisoning and spread of infectious foodborne diseases have always been one of the important medical challenges around the world, and many people experience food poisoning annually (2). Generally, milk and pastry products are major causes of food poisoning (3). With regard to their constituents and preparation and preservation conditions, pastries have a great potential to be contaminated by pathogenic and corrosive microorganisms.
Escherichia coli, as a normal flora of the intestine, colonizes the gastrointestinal tract of human and mammalian infants within a few hours after birth and plays a vital role in the physiological performance of digestive system. It should be noted that some E. coli strains become pathogenic by exploiting virulence factors through transferable genetic factors, such as plasmids, bacteriophages, and pathogenicity locusts. Generally, different E. coli serotypes are a major cause of diarrhea in developing countries or countries with poor health conditions (4).
The complications of E. coli infection include bloody and watery diarrhea and hemorrhagic colitis. Hemolytic uremic syndrome is also reported following the progression of pathogenicity and infection, especially by E. coli O157:H7 (5). Generally, E. coli is one of the important pathogens, which has shown increased resistance to most antibiotics (6). Some bacteria causing diarrhea in children include Campylobacter, Salmonella, Shigella, and E. coli (7). E. coli is also known as one of the causes of gastroenteritis in infants and children below five years, accounting for more than 60% of diarrhea cases in high-risk regions (8).
With regard to the frequent consumption of pastry cream in our community, microbial control is necessary from both health and industrial perspectives in order to preserve and increase the shelf-life and quality of food products. The occurrence of cream contamination in pastries is often similar to or even higher than that of pastry itself. Microbial contamination is one of the most common causes of transmission of digestive diseases to humans. The associated diseases also depend on cream preparation and preservation methods, community’s dietary habits, observation of hygiene standards by suppliers, and primary and secondary contamination of pastry cream (9).
Considering the high incidence of microbial contamination in pastry cream samples, consumers’ health may be at risk, and contamination of products can result in significant economic losses. With regard to the importance of this issue and control of microbial contamination in food products, development of contamination control strategies, observation of basic hygiene principles, proper training of workers, and more accurate monitoring by organizations responsible for production and supply seem essential.
2. Methods
2.1. Samples
In this cross-sectional study conducted in 2015 - 2016, a total of 350 pastry cream samples were randomly selected from confectionery suppliers in different districts of Hamadan and transferred in ice packs (4°C) to the food hygiene laboratory under sterile conditions.
2.2. Isolation and Identification of E. coli
The samples were homogenized, and 10 g of each sample was diluted in 90 mL of 0.1% sterile peptone water. E. coli was identified after culturing the samples in a nutrient-broth-enriched medium and solid media, such as nutrient agar (NA; Merck), violet red bile agar (VRBA; Merck), and eosin methylene blue agar (EMB; Scharlau, Spain), to identify bacteria isolated by the linear method; the samples were then incubated at 37°C for 24 to 48 hours. Finally, the antibiotic resistance pattern of isolated E. coli was determined, using standard disk diffusion method for 10 different antibiotics.
The Mueller-Hinton agar (MHA) plate was swabbed with nutrient broth (NB), inoculated with E. coli, and incubated to a turbidity of 0.5 McFarland standard (10). The antibiotic-sensitivity discs were placed on MHA agar (Merck), and the agar plates were incubated for 18 ± 2 hours at 36°C. The employed antibiotic disks included gentamicin, streptomycin, nalidixic acid, cefixime, chloramphenicol, ciprofloxacin, tetracycline, oxacillin, vancomycin, and penicillin. According to the findings, three sensitive, semi-sensitive, and resistant groups were classified with respect to the size of inhibited growth around the disk.
3. Results
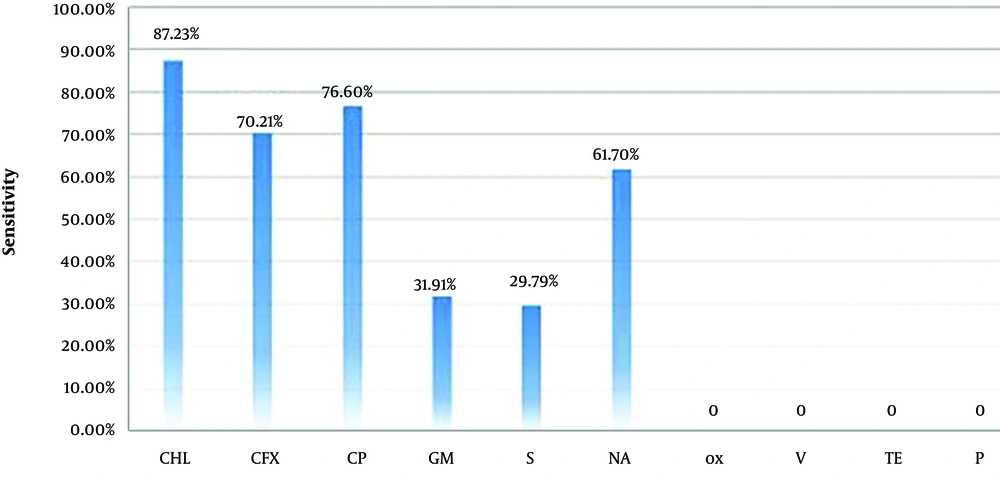
Based on the findings, 47 out of 350 samples contained E. coli. The results of antibiotic tests showed the highest rate of resistance to tetracycline, vancomycin, oxacillin (100%), and penicillin (72.34%) and the highest rate of sensitivity to chloramphenicol (87.23%), ciprofloxacin (76.59%), and nalidixic acid (61.70%). Reactions to other antibiotics are presented in Table 1 and Figure 1.
| Antibiotics | Sensitive | Semi-Sensitive | Resistant |
|---|---|---|---|
| Chloramphenicol | 41 (87.23) | 5 (10.64) | 1 (2.13) |
| Cefixime | 33 (70.21) | 0 (0) | 14 (29.79) |
| Ciprofloxacin | 36 (76.60) | 4 (8.51) | 7 (14.89) |
| Gentamicin | 15 (31.91) | 23 (48.94) | 9 (19.15) |
| Streptomycin | 14 (29.80) | 28 (59.57) | 5 (10.63) |
| Nalidixic acid | 29 (61.70) | 12 (25.53) | 6 (12.77) |
| Oxacillin | 0 (0) | 0 (0) | 47 (100) |
| Vancomycin | 0 (0) | 0 (0) | 47 (100) |
| Tetracycline | 0 (0) | 0 (0) | 47 (100) |
| Penicillin | 0 (0) | 13 (27.66) | 34 (72.34) |
aValues are expressed as number of isolates tested (%).
4. Discussion
Due to population growth, there is an urgent need for food control around the world. The prevalence of diseases, caused by the consumption of contaminated food, has always been one of the major problems in different communities, as elimination of these diseases, especially in countries with a poor health status, is costly (11).
Confectionery products (especially pastry cream) as nutritious foods are susceptible to the growth and multiplication of different microorganisms and transmission of microbial agents, causing food poisoning in consumers. The present findings and similar studies conducted in different parts of Iran and the world confirm the high risk of microbial contamination in such products, besides contamination transmission to consumers (12-14).
Overall, sources of contamination in pastry, especially pastry cream and sweet rolls, are as follows: Primary contamination of milk; use of local and non-pasteurized cream; non-compliance with the freezing cycle during storage; contamination of equipment, such as knives and cutting tools; improper manipulation by individuals working at confectionery workshops; poor personal hygiene of workers; and bacteria transmission from the staff hands and noses during processing and transportation phases (15, 16). Regarding the type of constituents and preparation and design methods, pastries are more likely to be contaminated by pathogenic agents, such as Enterobacteriaceae (17).
The results of the present study showed that 47 out of 350 pastry cream samples contained E. coli, which is consistent with the results of previous studies conducted in other cities of Iran on the contamination of pastry cream with E. coli. In this regard, Nikniaz et al. in a study from Tabriz, Iran, reported the rate of pastry cream contamination with coliform bacteria and E. coli to be 38.8% and 48.8%, respectively (3). In another study by Soltan Dallal et al. in Tehran, Iran, it was shown that the rate of E. coli contamination was 4.2% in pastry cream (18).
E. coli is one of the pathogens resistant to the majority of antibiotics, causing health problems in different countries (19). Few studies in Iran have investigated the antibiotic resistance of E. coli in food products. The research carried out by Saenz et al. indicated the highest antibiotic resistance of E. coli to nalidixic acid (53%) (20). This finding is not consistent with the findings of our study, as the rate of resistance was estimated at 12.27% in our study, which can be explained by geographical differences of these studies.
Moreover, in a study by Molaabaszadeh et al. on E. coli isolates from traditional ice cream in Khoy, Iran, the highest and lowest resistance rates were 82.98% and 12.76% to oxacillin and ciprofloxacin, respectively. Similarly, in the present study, the highest resistance rate was 100% to oxacillin, and the lowest resistance was 89% to ciprofloxacin. In addition, Molaabaszadeh et al. reported antibiotic sensitivities of 40.43% and 61.7% to tetracycline and gentamicin, respectively, while resistance to these antibiotics was 55.31% and 25.54%, respectively (21).
On the contrary, in the present study, the rates of sensitivity to tetracycline and gentamicin were 0% and 31.91%, respectively, while the rates of resistance to tetracycline and gentamicin were 100% and 19.15%, respectively. In another study by Mahdavi et al. on E. coli isolated from traditional cheese in Maragheh, Iran, the rates of sensitivity to chloramphenicol and streptomycin were estimated at 84.35% and 31.2%, respectively (22). These results are in agreement with the findings of the current study, as the corresponding rates were 23% and 29.8% in our study, respectively.
Additionally, in the study by Molaabaszadeh et al. on E. coli isolates from urinary tract infections in Tabriz, Iran, the rates of sensitivity to gentamicin and nalidixic acid were estimated at 37.98% and 52.1%, respectively (21). These results are similar to the findings of the present research. Moreover, in a study by Bonyadian et al. on E. coli isolates from raw milk and pasteurized cheese in Shahrekord, Iran, the resistance rates of E. coli to streptomycin and ciprofloxacin were reported to be 25.4% and 25.4%, respectively (23). The corresponding rates in the present study were reported to be 10.63% and 14.89%, respectively. Considering multiple reports on the transmission of resistant E. coli from dairy products to humans (24) and similarity of antibiotic resistance of E. coli in animal products and human infections, antibiotics should not be overprescribed (25).
4.1. Conclusions
According to the present findings and similar research in Iran, E. coli is extremely resistant to antibiotics. The major sources of this type of food contamination include microbial contamination of raw materials, such as cream, poor personal hygiene of workers, and microbial contamination of utensils used for pastry production. Therefore, all these factors can influence the final product and cause contamination. To avoid the contamination of pastry cream with bacteria, some techniques, including cold chain management, employee personal hygiene, use of healthy raw materials, and proper thermal processes, should be adopted.