1. Background
Quercetin (3, 3, 4, 5, 7-pentahydroxy flavone) is a plant flavonoid that is found in the daily diet of millions of people around the world (1, 2). Tea, vegetables, and red wine are rich in quercetin (3, 4). Quercetin is a free-radical scavenger and can temporarily bind to metal ions (5). Also, it has the ability to cross the blood-brain-barrier (6). Several studies have shown that quercetin has beneficial effects on many diseases such as respiratory and cardiac disorders and viral infections (7-9). In addition, it has been shown that quercetin can inhibit the growth of cancer cells (10, 11), as shown in clinical trials (12). Millions of people are suffering and dying of cancer every year (13). Although quercetin exhibits prooxidant activity at lower concentrations, its activity is reversed to antioxidant at higher concentrations (14).
Many unnecessary amino acids are essential for the growth of rapidly dividing tumor cells and to support and regulate the synthesis of nucleotides, macromolecule biosynthesis, cellular antioxidant systems, epigenetic changes, post-translational modifications, and many other cellular functions (15-18). Arginine is one of the conditionally essential amino acids for humans, and it has been shown that cancer cells are deficient in arginine (19). Also, it has been shown that tumor cells are sensitive to the absence of arginine, which provides a therapeutic strategy for eradicating cancer cells (20, 21). Arginine metabolism is interrelated with urea metabolism, synthesis of polyamines (putrescin, spermine and spermidine), as well as pyrimidine and nitric oxide biosynthesis. Besides, arginine metabolism has been associated with the development of many tumors (22-24). Changes in arginine metabolism might increase the mutation load of cells and alter cellular proliferation (25).
Human embryonic kidney 293 (HEK293) is a human cell line derived from embryonic kidney cells (26). It is used as a cellular model to study the effects of different substances on cancer cells (27). This cell line may also be extracted from the immature neonatal neurons of human kidney cells (28).
2. Objectives
In this study, we aimed to investigate the gene expression of a number of key enzymes involved in arginine metabolism in the HEK293 cells treated with quercetin.
3. Methods
3.1. Cell Culture and Quercetin Treatment
Human embryonic kidney 293 cells were acquired from the Iranian Biological Research Center, Karaj, Iran, and stored at 37°C under 5% CO2 and appropriate humidity. These cells were preserved in a medium containing 89% high-glucose DMEM (Gibco, USA), 10% fetal bovine serum (FBS) (Gibco, USA), and 1% penicillin 100 μg/mL and streptomycin 100 U/mL and cultured in 6-well cell culture plates. After reaching 70% confluence, the cells were treated with 57.5 and 115 µM quercetin (Sigma, USA) for 24 hours. Also, a set of untreated cells was used as control.
3.2. Cell Viability Assay
Human embryonic kidney 293 cells were cultured in a 96-well plate and incubated for 24, 48, and 72 h in a CO2 incubator before being exposed to different concentrations of quercetin (320, 160, 80, 40, 20, 10, 5, 2.5 and 1.25 µM). Then the cells were kept in the CO2 incubator for 24 h. After that, MTT reagent (4 - 5 mg/mL in sterile PBS) (Sigma, USA) was added to the wells, and incubation continued for another 4 h. Next, dimethyl sulfoxide (DMSO, Sigma, USA) was added to the wells which were then shaken. Optical density was read at 580 nm (29).
3.3. RNA Extraction and cDNA Synthesis
After 24 hours of treatment with 57.5 and 115 µM quercetin, RNA was extracted (blood/cultured cell total RNA mini kit, Favorgen Biotech) from HEK293 cells. The quality of extracted RNA was evaluated by a nanodrop (Nabi, Korea). The extracted RNA was then exposed to DNase I enzyme (Sinaclon, DNase I. RNase-free), and cDNA was synthesized (Roje technology, nRT-ROSET Kit).
3.4. Real-time PCR
Complementary DNA was used as a template for gene expression analysis. Using Oligo 7, IDT (integrated DNA technologies) and NCBI primer blast software, primers were designed for the genes of the enzymes involved in arginine metabolic pathways (Table 1), and the specificity of the designed primers was also evaluated (Table 1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18s rRNA genes were considered as internal controls. Quantitative expression of the genes was evaluated by the SYBR Green method [real QPCR 2x mix (Green), amplicon] (first cycle: 95°C for 15 minutes followed by 40 cycles: 25 seconds at 95°C and 60 seconds at 60°C). Gene expression was analyzed using a real-time PCR instrument (LightCycler® 96 Instrument, Roche).
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (NM-001256799.3) (30) | 5’-CCTGCACCACCAACTGCTTA-3’ | 5’-CATGAGTCCTTCCACGATACCA-3’ |
| 18s rRNA (31) | 5’-GTAACCCGTTGAACCCCATT-3’ | 5’-CCATCCAATCGGTAGTAGCG-3’ |
| Peptidylprolyl isomerase A (NM-001300981.2) | 5’-GGGTTTATGTGTCAGGGTGGT-3’ | 5’-ATGGACAAGATGCCAGGACC-3’ |
| Arginase 1 (NM-000045.4) | 5’-CTGAGGGTTGACTGACTGGA-3’ | 5’-TCTCAAGCAGACCAGCCTTTC-3’ |
| Arginase 2 (NM-001172.4) | 5’-TGATAGGAGCCCCGTTCTCAC-3’ | 5’-ACTGGAGAGCCTTTTCATCAAGC-3’ |
| Agmatinase (NM-024758.5) | 5’-CCTTCCAGTCCCTCATGGTTG-3’ | 5’-GTGATCTCCACCCAAGGTCAG-3’ |
| Arginine decarboxylase (NM-001293562.2) | 5’-AGCCTTGGACCTGTACTT-3’ | 5’-TCTAGCAGAACCTCCTTCTT-3’ |
| Ornithine carbamoyltransferase (NM-000531.6) | 5’-GGCTGTCAGATTTGTACCATCC-3’ | 5’-TTGTTCCCATCCCCGATCCAG-3’ |
| Spermidine synthase (NM-003132.3) | 5’-ATCCTCGTCTTCCGCAGTAA-3’ | 5’-TTGGCGATCATCTCCTGGTA-3’ |
| Spermin synthase (NM-001258423.1) | 5’-CCTCACTATGGCAGCAGCA-3’ | 5’-GCTCCTGGAAAATGGACTGGA-3’ |
| Nitric oxide synthase 1 (NM-000620.5) | 5’-GCAACACCCCTCTCTTGGAC-3’ | 5’-CAAAGTTTCTGCTGCGTGCTC-3’ |
| Argininosuccinate lyase (NM-000048.4) | 5’-CCACTGGCGTCATCTCTACG-3’ | 5’-GAATGGCATCCCTTTGCGG-3’ |
| Argininosuccinate synthase 1 (NM-000050.4) | 5’-AGCAGGCACCATCCTTTACC-3’ | 5’-CACTTTCCCTTCCACTCGCT-3’ |
| Ornithine decarboxylase 1 (NM-001287188.2) | 5’-TTGCGGATTGCCACTGATGAT-3’ | 5’-TCAGAGATTGCCTGCACGAAG-3’ |
3.5. Statistical Analysis
Statistical quantitative gene expression analysis of arginine metabolic enzymes was performed using REST 2009 (Corbett research) and GraphPad Prism 8 software packages. The Student t-test was used for statistical analysis, and a P-value of < 0.05 was considered as the significance level. Regression analysis was used to evaluate the primers’ efficiency scores.
4. Results
Herbal remedies, medicinal plants, and their ingredients are among the agents employed to fight cancer. Today, numerous researchers around the world are investigating the effects of these plant ingredients on cancer cells (32, 33). One of these plant ingredients is quercetin which is a flavonoid with proven anticancer properties (34, 35). Arginine is a conditionally essential amino acid for humans; however, many cancer cells are deficient in arginine. Therefore, this amino acid becomes essential to these cells (36). In this research, MTT assay showed that the concentrations of 2.5 μM to 1.2 mM of quercetin did not exert any toxicity against HEK293 cells. Therefore, we exposed HEK293 cells to 57.5 and 115 µM quercetin concentrations and observed altered gene expression of some of the studied arginine metabolic enzymes. These cells were treated with these concentrations of quercetin for 24 hours.
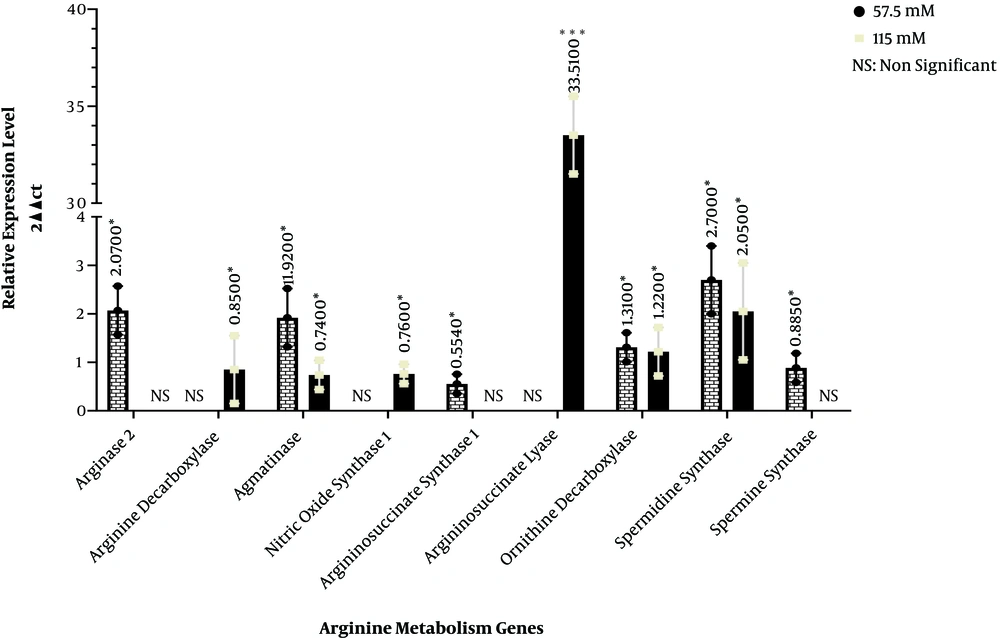
In arginine metabolism, ornithine carbamoyltransferase converts L-ornithine and carbamoylphosphate into L-citrulline in mitochondria. This enzyme had no expression in neither untreated HEK293 cells nor the cells treated with 57.5 and 115 µM quercetin. Arginase I and II convert L-arginine to L-ornithine. Arginase 1 enzyme is located in cytoplasm while arginase 2 is found in mitochondria. Arginase 1 showed no expression in the HEK293 cells treated with 57.5 and 115 µM quercetin compared with the control group. On the other hand, while arginase 2 expression did not significantly change in 115 µM quercetin concentration compared with the control, at 57.5 µM quercetin concentration, the gene expression of this enzyme showed a 2.07-fold increase. Arginine decarboxylase enzyme converts L-arginine into agmatine in mitochondria. Although the gene expression of this enzyme in the HEK293 cells treated with 57.5 µM quercetin was not significantly different compared with the control group, at 115 µM concentration, the gene expression of this enzyme showed a 0.85-fold increase. The agmatinase enzyme, which is expressed in mitochondria, converts agmatine into putrescine. The HEK293 cells exposed to 57.5 µM quercetin showed a 1.92-fold increase in the gene expression of this enzyme while at the 115 µM quercetin concentration, a 0.74-fold decrease was seen in agmatinase gene expression compared to the control group. Nitric oxide synthase 1 catalyzes the conversion of L-arginine into nitric oxide and L-citrulline in cytoplasm. In quercetin-treated HEK-293 cells, while the 57.5 µM concentration did not significantly change the gene expression of this gene in comparison with the control group, the HEK293 cells treated with 115 µM quercetin showed a 0.76-fold decrease compared with the control group. Argininosuccinate synthase 1, a cytoplasmic enzyme, converts L-citrulline into argininosuccinate. In the HEK293 cells exposed to the 57.5 µM quercetin concentration, the expression of this enzyme showed a 0.554-fold decrease; nonetheless, at the 115 µM concentration, there was no significant difference between treated and untreated groups. Argininosuccinate lyase, which is also located in cytoplasm, converts argininosuccinate into L-arginine. Although the gene expression of this enzyme did not show significant changes in treated and untreated cells at the 57.5 µM quercetin concentration, the expression of this enzyme at the 115 µM quercetin concentration showed a 33.513-fold increase. The conversion of L-ornithine to putrescin is mediated by ornithine decarboxylase in cytoplasm. At 57.5 and 115 µM quercetin concentrations, the gene expression of this enzyme increased 1.31- and 1.225-fold, respectively. In another enzymatic reaction in cytoplasm, spermidine synthase converts putrescin to spermidine. In the HEK293 cells treated with 57.5 and 115 µM quercetin, the expression of this enzyme increased by 2.7- and 2.05-fold, respectively, compared to the control group. Spermine synthase also catalyzes the conversion of spermidine to spermine in cytoplasm. In the HEK293 cells treated with 57.5 µM quercetin, the expression of this enzyme showed a 0.885-fold decrease. However, at the 115 µM concentration, the expression of this enzyme was not significantly different between quercetin-treated and untreated cells (Figure 1).
5. Discussion
Cancer is one of the leading causes of death worldwide, that affects the lives of millions of people every year (13). A lot of research is underway to fight with this disease (37-39). Arginine is one of conditionally essential amino acids for normal human cells and is involved in the synthesis of many tumor-inducing metabolites in cancer cells, influencing their proliferation, growth, and metastasis (40, 41). Various types of cancer cells may be either arginine dependent or independent (42). Arginine metabolism involves the conversion of L-arginine to L-ornithine by arginase I and II enzymes. Arginase I is a cytosolic enzyme in liver cells. Arginase II; on the other hand, is a mitochondrial enzyme mostly expressed in non-liver cells (43). Although arginase I was not expressed in HEK293 cells, the expression of arginase II significantly increased in the HEK293 cells treated with quercetin at the 57.5 µM concentration. Arginine metabolism is involved in the production of putrescin, spermine, and spermidine polyamines (44). Three enzymes including ornithine decarboxylase 1, spermine synthase, and spermidine synthase catalyze the enzymatic reactions leading to the production of these polyamines. Oncogenes and free polyamine content play a significant role in determining the amount of ornithine decarboxylase 1, spermine synthase, and spermidine synthase proteins (45, 46). In this study, the gene expression of these enzymes significantly decreased in the HEK293 cells exposed to the 115 µM quercetin concentration compared to the cells treated with the 57.5 µM concentration of this flavonoid. This indicates the anti-tumor effects of quercetin by reducing the synthesis of the catalytic enzymes involved in arginine metabolism. On the other hand, arginine decarboxylase that catalyzes the synthesis of agmatine from L-arginine can reduce the production of polyamines by inhibiting ornithine decarboxylase 1 (47). The gene expression of this enzyme showed a significant increase in the HEK293 cells treated with 115 µM quercetin. So, the decreased expression of polyamines observed in this study could be attributed to the action of an ornithine decarboxylase 1-independent pathway. Previous studies on various cell lines have shown that quercetin reduces ornithine decarboxylase (ODC) expression. However, this study showed that the expression of this enzyme increased in quercetin-treated HEK 293 cells (48). Also, nitric oxide synthase 1 plays an important role in arginine metabolism. In human cells, the three enzymes of inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and endothelial nitric oxide synthase (eNOS) are involved in the conversion of L-arginine to nitric oxide and L-citrulline, and each of these enzymes has its own characteristics (49). In this study, nNOS, which is expressed in neurons, was upregulated in the HEK 293 cells treated with 115 µM quercetin. This enzyme can be involved in carcinogenesis as well (49-51). In another study on rat models of arterial erectile dysfunction, it was shown that quercetin treatment had no effect on nNOS expression (52).
Argininosuccinate synthase 1 and argininosuccinate lyase are two enzymes converting citrulline to arginine. Argininosuccinate lyase plays a vital role in the growth of various tumor cells and has been suggested as a therapeutic target for treating cancers (53-55). This enzyme significantly increased in the HEK 293 cells treated with 115 µM quercetin. The argininosuccinate synthase 1 enzyme exhibits variable expression in different cancers (42). In this study, argininosuccinate synthase 1 showed a significant increase in the HEK 293 cells exposed to 57.5 µM quercetin. Consistent with our findings, the hyperammonemic rats fed with 50 mg/kg body weight quercetin showed overexpressed ASS1, OTC, and ARG (56).
5.1. Conclusion
To the best of our knowledge, this is the first study investigating arginine metabolic enzymes in the HEK293 cells treated with quercetin. It was shown that quercetin could change the gene expression of the enzymes involved in arginine metabolism. The findings of this study suggest investigating the role of plant flavonoids in future cancer therapy research.