1. Background
Tissue adhesion formation is a prevalent and serious problem that occurs within four to six days after most abdominal surgical procedures (1). There is a classical concept that proposes a reduction in peritoneal fibrinolytic activity as a result of postoperative adhesion formation (2). Postoperative adhesion leads to serious complications such as infertility, ectopic pregnancy, bowel obstruction, and abdominal and pelvic pain (3). Postoperative adhesion mostly occurs after general surgical abdominal operations and open gynecologic pelvic procedures. Studies have shown the incidence of postoperative adhesion ranges from 67 to 93% after general surgical abdominal operations and up to 97% following open gynecological pelvic procedures (4). Adhesions lead to complications such as pain and bowel obstruction; thus, a second operation called adhesiolysis is required for breaking the adhesions (5), which, in turn, imposes risks including blood loss, injury to bladder and bowel, and infertility (6). Fibrinolytic agents, anticoagulants, anti-inflammatory agents, and antibiotics are used to alleviate this problem (7). However, these agents alone are not effective to solve the problem because their clearance occurs rapidly (8). In addition, a minimally invasive surgery can help minimize the probability of adhesion formation although it is not always appropriate or applicable. In a study in 1994 in the U.S, surgeon expenditure imposed by adhesiolysis was $1.3 billion (9). Although the mechanism underlying adhesion formation is not fully understood, it has been shown that abdominal surgery if causes damage to the peritoneum results in the activation of the surrounding mesothelium and leads to the release of local inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) into the abdominal cavity. Consequently, immune cells including neutrophils, macrophages, and eosinophils infiltrate into the peritoneum. This process is associated with the increase of oxidative stress leading to more infiltration and subsequent activation of neutrophils and macrophages. Finally, this inflammatory process leads to the massive secretion of extracellular matrix molecules, including fibronectin, hyaluronic acid, glycosaminoglycans, and proteoglycans by fibroblasts and myofibroblasts, which can cause the formation of fibrinous adhesions between peritoneal surfaces (10, 11).
M. sylvestris L. known as mallow is a genus belonging to the family Malvaceae with shallowly lobed leaves and purple flowers. It is grown in North Africa and South-west Asia (12). Based on the phytochemical analysis, it contains active compounds such as polysaccharides, anthocyanins, coumarins, tannins, flavones, flavonols, anthocyanidines , leucoanthocyanidines , mucilage, terpenoids, and essential oil (13). The extract of M. Sylvestris has exhibited wound healing activity, as well as anti-oxidant and anti-inflammatory properties (14). Prudente et al. demonstrated the hydroalcoholic extract of M. sylvestris could suppress acute inflammatory responses in mice skin, inflammatory cytokine production, oedema formation, and neutrophils migration (13). In another study, M. sylvestris showed protective effects against vanadium-induced damage in rat kidneys by the decrease of lipid peroxidation and alteration of antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase (15).
2. Objectives
In this study, we aimed to investigate the effects of the hydroalcoholic extract of M. sylvestris on postoperative peritoneal adhesion in rats.
3. Methods
3.1. Chemicals
We used both aqueous and alcoholic extracts of M. sylvestris flowers in this study. IL-1β and TNFα rat serum ELISA kits were purchased from RayBio Company. All other solvents and chemicals were of analytical grade and purchased from Merck (Darmstadt, Germany) or Sigma Aldrich (Sigma, St Louis, MO).
3.2. Preparation of M. sylvestris Flower Extracts
M. sylvestris was collected from Mashhad, Razavi Khorasan province, Iran. 50 g of the powdered plant was extracted by Ethanol 50%. The obtained extract was evaporated in low pressure at 50°C. The dry extract was weighed and stored in a refrigerator.
Based on the traditional medical literature, the medicinal features of Malva sylvestris is related to the leaves and flowers of the plant. We used the flowers of the plant in our study. Initially, plant flowers were dried in shade at the room temperature. Then, it was powdered using an electric mill to have more contact with the solvent. The particles were smaller than 1.8 inches. The maceration procedure was used for extraction. Initially, the powder was put in a container. Then, water for preparation of aqueous extract, alcohol (ethanol 96°) for preparation of alcoholic extract, and a mixture of water and alcohol (at a ratio of 70%:30%) for preparation of hydroalcoholic extract were added to separate containers. After constant stirring for 2 to 4 days, the resulting mixture was filtered and the liquid was evaporated using a rotary evaporator to obtain a concentrated extract. The aqueous extract, hydroalcoholic extract, and alcoholic extract were used freshly on the day of the experiment.
3.3. Animals
Male Wistar rats weighing 250-300 g were housed in ventilated rooms in standard conditions (24 ± 2°C temperature, 12-h light/dark cycle, and 60 ± 5% humidity). They were provided with food and water ad libitum. The animals were housed for one week before the experiments to acclimatize to new condition.
3.4. Surgical Procedure
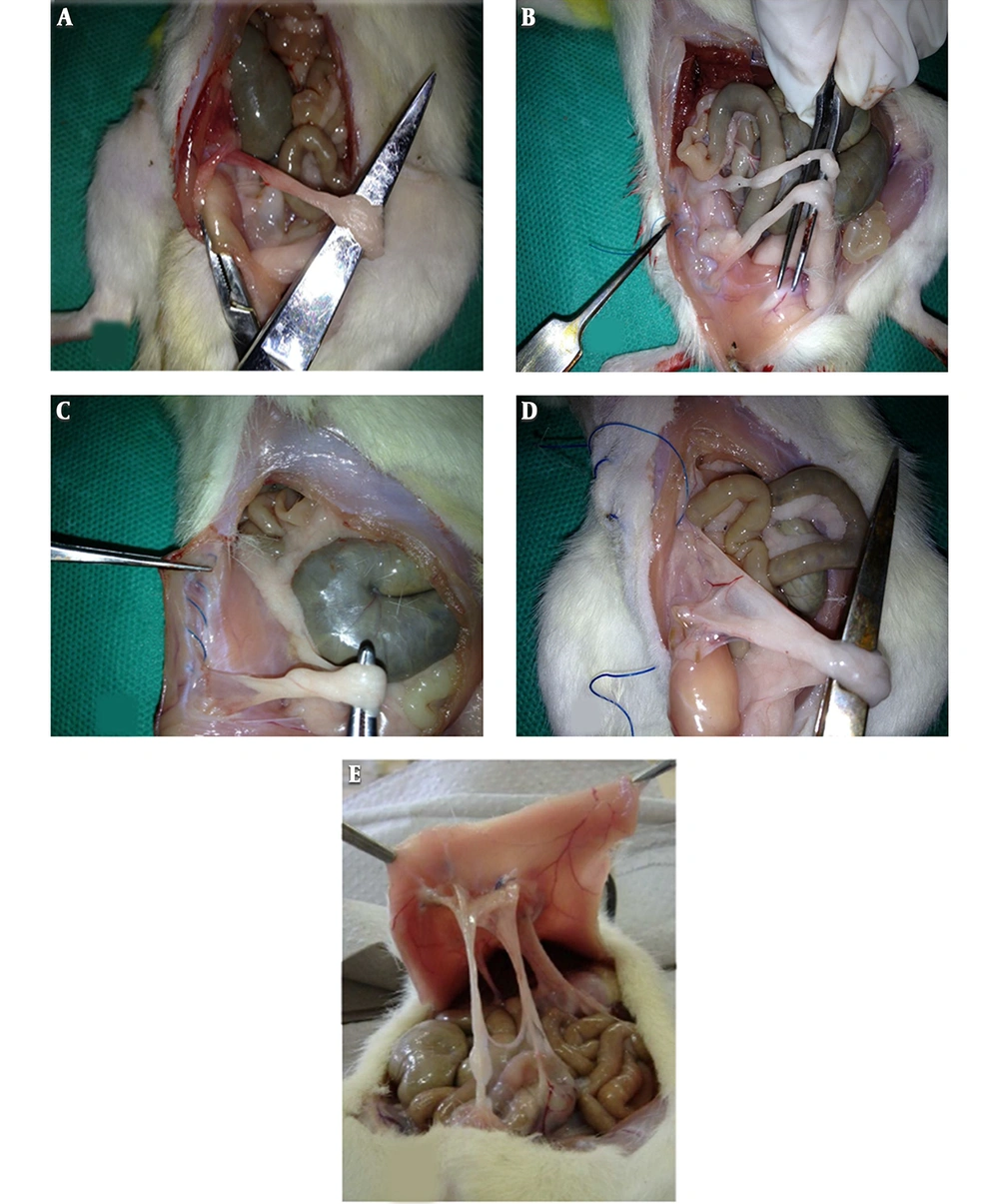
The study protocol was approved by the local Animal Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran, in accordance with the Guide for the Care and Use of Laboratory Animals. Abdominal adhesion was created in animals using a model and a procedure described previously (16, 17). Briefly, rats were anesthetized using an intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The intraperitoneal injections were done by sterile, isotonic, and nonirritating materials to prevent probable subsequent adhesion formation as much as possible (18). The abdomen surface was shaved and disinfected with alcohol and povidone-iodine solution preoperatively. After drying, a 3-cm midline incision was performed and the abdomen was opened. The adhesion formation model was applied using the peritoneal button creation (PBC) technique. According to this model, 2.0 Prolene sutures were used to create four peritoneal buttons on the parietal peritoneum in a chain distribution. Each suture encapsulated approximately 2 cm of parietal peritoneum. The diameter of the tied suture was approximately 5 mm. By using a continuous 6 - 0 polypropylene (non-absorbable) suture, the wound was then closed and the repair leak was tested with a simple pressure test. After the operation, the caecum was returned to the abdomen and the abdominal wall was closed with a 3 - 0 poly gelatin suture. The animals were allowed to recover in a warm, quiet, well-ventilated environment and proper analgesic (Flunixin meglumine (Banamine) 2.5 mg/kg SC) and antibiotic agents were used to minimize post-surgical complications (19).
3.5. Experimental Procedure
Twenty-four male Wistar rats were randomly assigned into four groups (n = 6 each) using the block randomization method (20). For Group 1 (control group), peritoneal adhesions were induced as described above, followed by saline solution administration. Group 2 received the aqueous extract of M. sylvestris flowers at 0.2 g dry mallow/kg body weight following adhesion induction. Group 3 received the ethanolic extract of M. sylvestris flowers at 0.2 g dry mallow/kg body weight following adhesion induction. Group 4 received the hydroalcoholic extract of M. sylvestris flowers at 0.2 g dry mallow/kg body weight following adhesion induction (21).
The rats were anesthetized and operated in order to create four sutures. Before the abdomen was closed, four nebulized solutions including saline solution, aqueous extract, ethanolic extract, and hydroalcoholic extract of M. sylvestris were applied for two minutes and then, the abdomen was sutured and the rats were transferred to the recovery room until they became conscious. Fifteen days later, first, 1 mL of blood sample was taken from the tails and the plasma was separated and kept at - 70°C. Then, the rats underwent the operation. A surgeon who was blinded to the treatment scored the adhesions using the macroscopic scoring system described by Liu et al. (8). Cecum and peritoneal samples were sent for histopathology assessment and adhesion formation severity. A pathologist blinded to the animal’s treatment investigated the histological changes of the adhesion under a light microscope and scored the specimen using a scoring system as previously mentioned (22, 23).
3.6. Determination of Inflammatory Cytokines
The levels of TNF-α and IL-1β in the plasma samples were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (RayBio Company) according to the manufacturer’s protocol.
3.7. Statistical Analysis
Statistical differences were evaluated by the One-way ANOVA, followed by Tukey post hoc test. For macroscopic and histological damage, data are presented as median and interquartile ranges. For the analysis of these factors, we used the Kruskal-Wallis test, followed by a multiple-comparison test (Dunn’s). A P value of < 0.05 was considered significant.
4. Results
4.1. Effects of M. sylvestris Extract on Macroscopic Peritoneal Adhesions
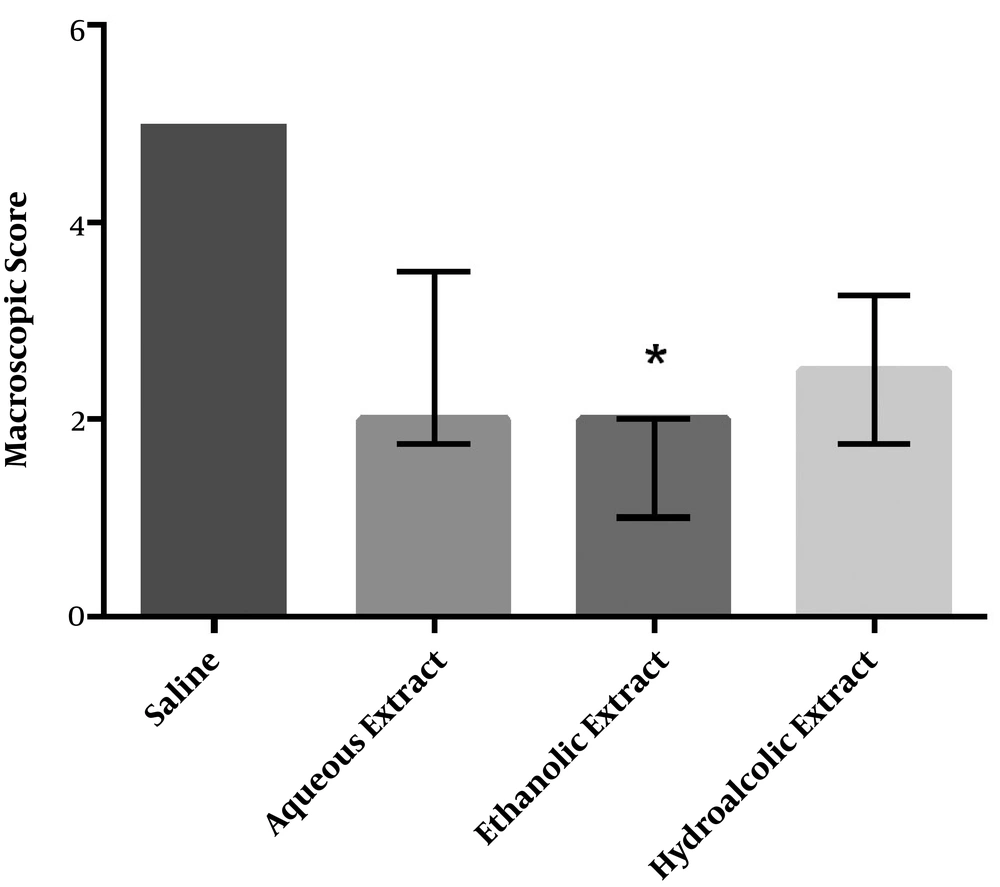
All animals survived after surgery and application of agents. 15 days after the induction of adhesion, all the animals were evaluated for the severity of adhesion formation and scored according to the criteria as shown in Figure 1. Although the intensity of adhesions was lower in all the three groups of different M. sylvestris extracts than in the control group, only the effect of the ethanolic extract on the macroscopic score was statistically significant (Figure 2).
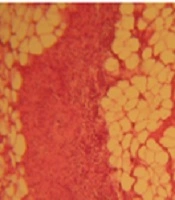
Adhesion was scored based on the following criteria: 0 = no adhesion; 1 = thin filmy adhesion, easily removable (A); 2 = more than one thin adhesion (B); 3 = thick adhesion with focal point (C); 4 = thick adhesion with planar attachment (D); 5 = extensive and thick vascularized adhesion or more than one planar adhesion (E); Figure 1E was adapted from Jomezadeh et al. (16) with permission.
Effect of M. sylvestris extracts on the macroscopic score of postoperative intra-abdominal adhesions. Treatment of rats with the ethanolic extract of M. sylvestris significantly reduced the severity of abdominal adhesions. Data are expressed as Median ± IQR. * P < 0.05 compared to the saline group.
4.2. Effects of M. sylvestris Extract on Histopathological Changes
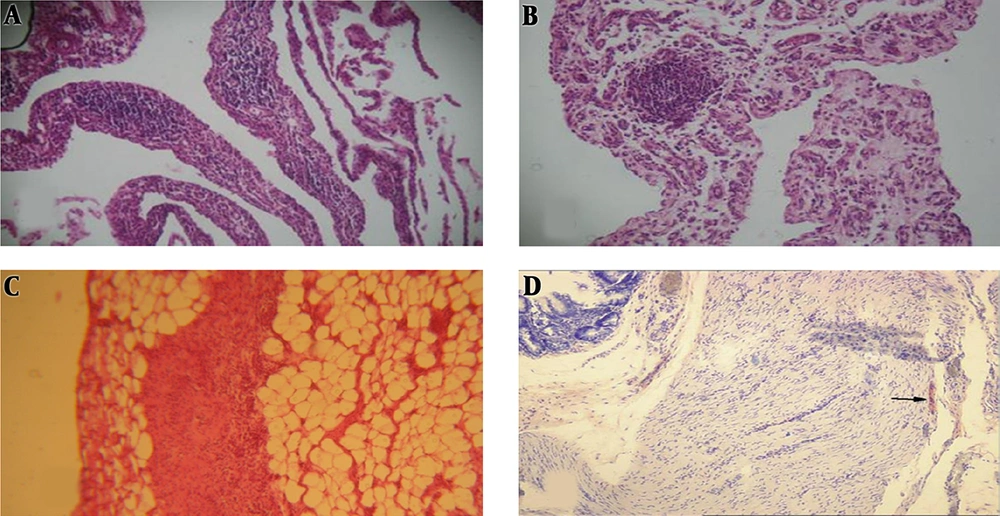
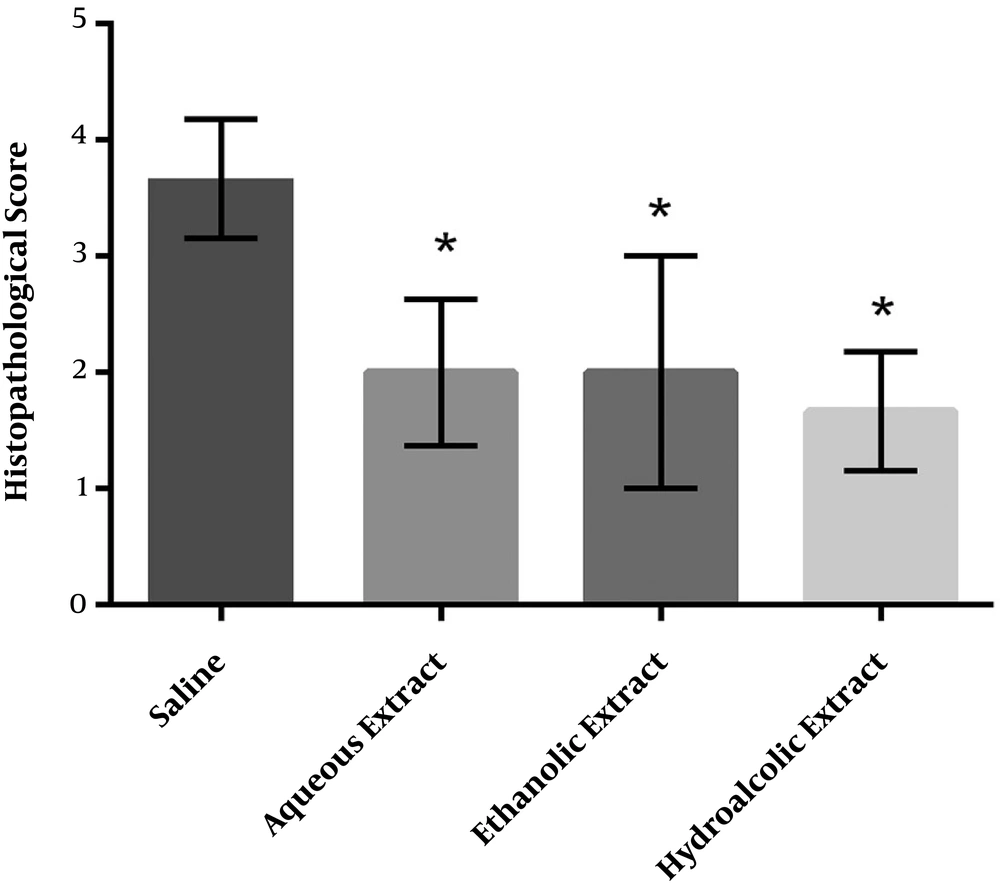
The histology results of the cecum and abdominal wall adhesion were paralleled to the macroscopic scores (Figure 3). Severe fibrosis and inflammation were observed in the cecum in the control group. All the three different M. sylvestris extracts significantly decreased the intensity of fibrosis and inflammation compared to the control group (Figure 4).
Histopathological examination performed according to the following criteria: inflammation stage: fibrinous inflammation, neutrophilic infiltration (A); proliferative stage: granulation tissue, edema, proliferation, and migration of connective tissue cells (B); scar formation: collagen and other ECM components deposition (C); fibrosis stage: excessive deposition of collagen and other ECM components (D); Figure 3D adapted from Karatas et al. (22) with permission.
Effect of M. sylvestris extracts on the microscopic score of postoperative intra-abdominal adhesions. Treatment of rats with various extracts of M. sylvestris significantly reduced the severity of abdominal adhesions from the microscopic point of view. Data are expressed as Median ± IQR. *P < 0.05 compared to the saline group.
4.3. Effects of M. sylvestris Extract on TNF-α and IL-1β Serum Concentration
The induction of intra-abdominal adhesions by surgery increased the serum levels of inflammatory cytokines in the control group (saline). However, M. sylvestris extract did not have any statistically significant effect on the serum levels of TNFα and IL-1β (Table 1).
| Extraction /Inflammatory Biomarkers | TNF-α (pg/mL) | P Value (Compared to Controls) | IL-1β (pg/mL) | P Value (Compared to Controls) |
|---|---|---|---|---|
| Control | 0.1 ± 0.01 | 0.13 ± 0.00 | ||
| Aqueous | 0.09 ± 0.02 | 0.99 | 0.13 ± 0.03 | 1.00 |
| Alcoholic | 0.14 ± 0.08 | 0.49 | 0.19 ± 0.06 | 0.17 |
| Hydroalcholic | 0.11 ± 0.04 | 0.95 | 0.16 ± 0.03 | 0.79 |
a The control group received the saline solution and other groups received aqueous, ethanolic, and hydroalcoholic extracts of Malva sylvestris.
bData were shown as means ± SD (n = 6); there was no significant difference between various groups (P > 0.05).
5. Discussion
Postoperative adhesion is a complication leading to morbidity in millions of people around the world (24). In this study, abdominal surgery resulted in the high level of TNF-α and IL-1β serum concentrations and a great extent of adhesion formation. The results of the present study indicated that the alcoholic extract of M. sylvestris could reduce intraperitoneal adhesion significantly. In addition, other extracts reduced fibrosis and inflammation. However, M. sylvestris extracts did not affect the TNF-α and IL-1β serum concentrations. Although the exact etiology of peritoneal adhesion remains elusive, the involvement of some pathways and factors has been proven. Peritoneal injury-induced inflammatory process involves different immune cells and inflammatory cytokines that eventually lead to collagen synthesis and fibrotic adhesion (25).
Furthermore, it has been suggested that reactive oxygen species (ROS) and reactive nitrogen species (RNS) may be overproduced following conditions such as surgical trauma, ongoing infections, or inflammation in injured peritoneal surfaces, causing normal fibroblasts to acquire the adhesion phenotype (3). The roles of ROS and RNS in this process were proven with respect to the palliative effect of antioxidants such as vitamin E and Lycopene on postoperative adhesion (26, 27).
Various effects of M. sylvestris such as anti-inflammatory, anti-microbial, and anti-oxidant features have been proven in many clinical and animal studies (21, 28-30).
Following damage to the peritoneal mesothelial cells, inflammation and healing process begin. Blood vessels dilate and their permeability increases. On the other hand, fibrin exudate covers the damaged area. Due to proper functioning, fibrinolysis will digest fibrin bonds between the damaged areas. Otherwise, fibroblasts invade the region, followed by the deposition of extracellular matrix and scar formation. It also multiplies the macrophages in the affected area. A series of inflammatory factors such as plasminogen, IL-1, IL-6, and TNF-α are secreted. These factors may protect fibroblasts against fibrinolytic. Eventually, the fibrin gel is organized and stickiness.
In a study conducted in 2010 by Pirbalouti et al., the anti-inflammatory and regenerative effects of M. sylvestris were investigated. They concluded that M. sylvestris causes faster contraction of wounds and decreases scars formation and inflammatory cells in the skin of rats with and without diabetes (21, 31).
In another study conducted by Barikbin et al., the efficacy of M. sylvestris in the treatment of eczema was proven due to its regenerative properties and few side effects. They found that it could be a good alternative for corticosteroids and histamines in therapies (32).
In another investigation by shale et al., the antibacterial effect of another edible herb in this family named M. parviflora (with hexane, methanol, and water extracts) on Gram-positive and Gram-negative bacteria was observed. Although the different parts (leaves and roots) of the plant showed various antibacterial features, the anti-inflammatory properties were alike. The hexane and water extracts had the most and least inhibition effects, respectively (33).
In another study, the antibacterial effect of cetylpyridinium chloride-based mouthwashes was determined against Staphylococcus aureus. It was observed that cetylpyridinium alone could inhibit three strains of Staphylococcus aureus while in combination with Malva, it inhibited the growth of all 28 strains, which demonstrated the synergistic effects of these agents (34).
A group of researchers compared the extracts of various parts of M. sylvestris such as leaves and fruits for their antioxidant nature and chemical ingredients. The leaves of this plant showed potent antioxidant features (radical scavenging activity, lipid peroxidation inhibition, and brain cells homogenates) and were a powerful source of antioxidants (phenols, flavonoids, carotenoids, and tocopherols) (35). A similar study was conducted on the plants used conventionally to treat inflammations to evaluate their anti-inflammatory, antioxidant, and antiradical properties. M. sylvestris was one of these edible plants that caused a notable effect on the inflammation reduction (about 21%) (36).
Significant increases in lipid peroxidation levels and superoxide dismutase, catalase, and glutathione peroxidase activities along with renal structural malformation were observed in rats’ kidneys exposed to ammonium metavanadate. The co-administration of M. sylvestris caused normal lipid peroxidation levels, antioxidant enzyme activities, and histological appearance compared to control rats. This high antioxidant capacity might be due to its richness in phenolic compounds (15).
Having phenolic and flavonoids compounds with anti-inflammatory properties besides high antioxidant power could be the reasons for the anti-inflammatory activity of the plant extract.
The inhibition of protein kinase C by flavonoids can cause lymphocyte malfunction. A special type of flavonoids named Quercetagetin also inhibits the phospholipase 2A enzyme. Flavonoids could also inhibit Na+/K+ ATPase, an enzyme that induces the release of histamine from mast cells.
Mallow plant flowers contain mucilage compounds, which are among the main components denoting the medicinal character of the plant (35). The hydrolysis of mucilage produces sugars such as arabinose, glucose, rhamnose, galactose, and galacturonic acid that probably are the internal agents causing mesothelial and peritoneal regeneration.
In our study, similar results (anti-inflammatory, anti-bacterial, and antioxidant properties) for three Mallow plant extracts were obtained, which caused a macroscopical reduction in the severity of peritoneal adhesions in addition to microscopic amelioration.
It has been proven that mucilage is dissolved in the aqueous extract and flavonoids are dissolved in the alcoholic extract. Considering, in our study, the aqueous and hydroalcoholic extracts of the plant were more effective in the treatment procedure, it can be concluded that the anti-inflammatory properties of M. sylvestris in the prevention of adhesion are more related to the presence of mucilage of this plant.
The antioxidant properties of M. sylvestris have been reported in several studies and in different pathological conditions (37, 38). Similar to these studies, our study demonstrated that M. sylvestris is a potent antioxidant for the significant reduction of postoperative adhesion.
Due to the three properties of M. sylvestris (regenerative, antimicrobial, and anti-inflammatory properties), the severity of adhesions and peritonitis reduced. In this investigation, since the concentrations of TNF-α and IL-1β did not decrease after treatment, it seems probable that the regenerative and anti-bacterial properties of M. sylvestris reduced the severity of adhesions in the peritoneum. In the aqueous and hydroalcoholic extract groups, macroscopic adhesion intensity reduced significantly when compared to the saline solution group. Nevertheless, in the alcoholic extract group, macroscopic adhesion intensity did not reduce significantly. Due to the reduction of adhesion in the histopathological study, the positive effect of M. sylvestris in abdomen surgery is obvious.
Since the concentrations of TNF-α and IL-1β did not decrease, it seems that the effect of the extract was local and it could not reduce the systemic inflammatory factors. As another explanation, the amount of M. sylvestris might be less than the effective concentration on inflammatory factors. However, adhesion intensity decreased in all three groups compared to the saline solution group, and the decreases were significant.
Therefore, we suggest various adhesion induction methods and higher concentrations of M. sylvestris be studied in future research.
5.1. Conclusions
The effect of M. sylvestris on postoperative adhesion was studied for the first time. No deaths and specific side effects were observed for this medical herb in this study. M. sylvestris has a great potential in the removal of peritoneal adhesions and a significant reduction in the severity of adhesions.