1. Background
Chaerophyllum genus, from the Apiaceae family, includes nearly 110 species in temperate and sub-temperate regions of Asia, Africa, and Europe. Chaerophyllum (chervil) is a native herb with weak stems and leaves, similar to parsley or parsley-like leaves. C. macropodum is known as “Gazarsare” in Mahdishahr (Sangsar) and “Jafari Frangi Kohi” in Iran. Eight Chaerophyllum species have been found in Iran, including C. macropodum, which is a wild species endemic to Iran and Turkey. The leaves of this plant are used in the food industry, as well as medicine. Previous studies have confirmed the presence of phenolic acids and constituents with cytotoxic and antimicrobial activities in Chaerophyllum species (1-4).
Candida species, particularly Candida albicans, are common pathogens in patients with organ transplantation, AIDS, and neoplastic disease, immunocompromised patients, and users of immunosuppressive drugs and broad-spectrum antibiotics. Recently, the use of medicinal plants has been highlighted, as natural products with therapeutic properties are cost-effective, available, and beneficial. In addition, chemical drugs have more side effects. Treatment of some diseases using medicinal plants, especially herbs, is in its infancy. On the other hand, researchers try to detect the active constituents of extracts to treat different diseases.
2. Objectives
We aimed to examine the anti-Candida and antioxidant potential of hydroalcoholic leaf extracts of C. macropodum via GC analysis.
3. Methods
3.1. Materials
Sigma-Aldrich supplied Gallic acid, trichloracetic acid (TCA), vitamin C, Quercetin, DPPH, Folin-Ciocalteu reagent, and butylated hydroxytoluene (BHT). The spectrophotometric assays were performed with a spectrophotometer (Analytik Jena, Germany).
3.2. Collection of Plants
After collecting C. macropodum leaves from Roudbarak in Mahdishahr (Semnan, Iran), they were confirmed by professional botanists. A voucher sample was deposited at the Research Center for Medicinal Plants. The dried samples were powdered for further analysis.
3.3. Extract Preparation
For extraction, the powdered leaf (50 g) was mixed with 6:3:1 ethyl acetate: methanol: distilled water (500 mL) overnight. The temperature was adjusted to 40°C in a Soxhlet system for eight hours before the evaporation solvent was incubated at room temperature.
3.4. Total Phenolic Content (TPC)
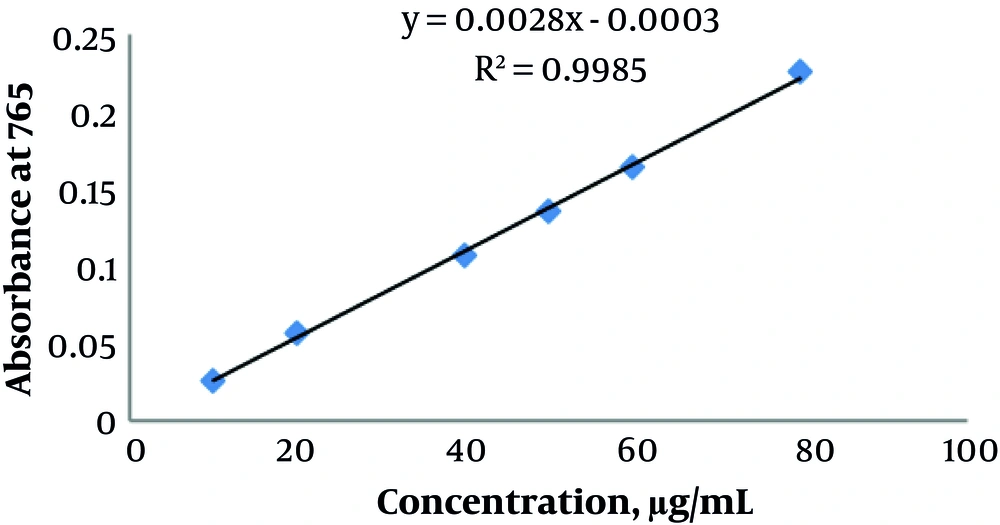
The Folin-Ciocalteu method was used according to the literature (5, 6) with some modifications to evaluate TPC. Following two hours of incubation, absorbance was read at 765 nm. For the measurements, the standard Gallic acid (GA) curve was applied, presented as grams of GA equivalent (GAE) per 100 g of dry weight (7).
3.5. Total Flavonoid Content (TFC)
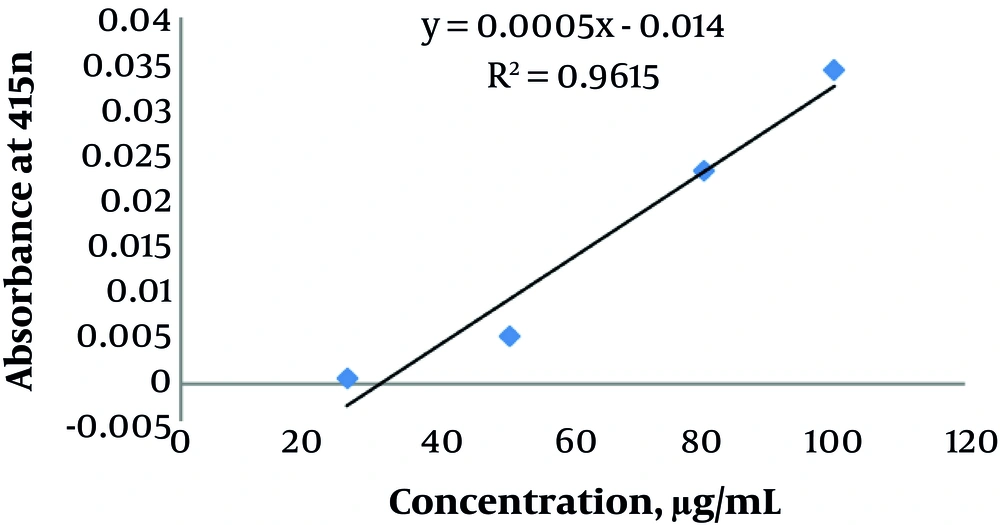
To determine TFC, the extract (1 mL) in methanol was mixed in dilutions of standard quercetin solution in a flask (10 mL) of distilled water (4 mL). During five minutes of incubation, 5% sodium nitrite (0.3 mL) was added. In addition, aluminum chloride (0.3 mL) was added before six minutes of incubation, followed by 1 M sodium hydroxide (2 mL). OD was determined at 510 nm against a blank reagent. TFC was expressed as mg of quercetin equivalent per gram of sample (8-10). To obtain the extract concentrations, the standard curve of quercetin was used. TFC was calculated as follows:
TFC = R × D.F × V/w
where R represents the standard curve result, D.F denotes the dilution factor, V indicates the stock solution volume, and W represents the plant weight.
3.6. Antioxidant Assay
For the antioxidant assay, after dissolving the sample in 95% methanol at 1 mg/mL, it was diluted for preparation of series concentrations. The reference chemicals were used for comparisons.
3.6.1. DPPH Radical Scavenging Assay (DRSA)
DRSA was conducted and the absorbance was read at 517 nm based on studies by Burits and Bucar (11), Cuendet et al. (12), and Kirby and Schmidt (13):
DPPH radical scavenging percentage: I% = (1 - As/Ac) × 100
where Ac indicates the control absorbance, while As denotes the sample solution absorbance.
3.6.2. Reducing Power Assay
For this purpose, the method proposed by Hinneburg et al. (14) was applied. To measure absorbance, a spectrophotometer was used at 700 nm after mixing the supernatant (2.5 mL) with FeCl3 (0.1%, 0.5 mL) and distilled water (2.5 mL). An increase in absorbance was indicative of increased reducing activity.
3.6.3. β-Carotene Bleaching Test (BCBT)
To prepare the stock solution of linoleic acid and β-carotene, linoleic acid (25 μL), as well as Tween 40 (200 mg), was added after dissolving β-carotene (0.5 mg) in chloroform (1 mL), since β-carotene is not considered water-soluble. Following that, 100 mL of oxygen-saturated distilled water was added during 30 minutes via vigorous shaking at 100 ml/min. Finally, the mixture (2500 μL) was added to tubes and then, the extract (350 μL) was added at 2 g/L.
OD was measured at 490 nm after incubation. A similar procedure was applied using a positive BHT control, along with a blank. Comparisons were made in terms of antioxidant potential between the extract, BHT, and the blank. All assays were conducted three times, and the relative antioxidant activity (RAA) was estimated as follows (15):
RAA = Asample/ABHT
3.7. Preparation of Microbial Strains and Inoculum
3.7.1. Serial Dilutions
For a final concentration of 400 μg/μL, 2 g of C. macropodum extract was dissolved in 5 mL of DMSO (16). To prepare serial dilutions of 400, 350, 300, 250, 200, 150, and 100 μg/μL, sterile distilled water was used. The reference strain was Candida albicans (ATCC10231). Gram-positive and Gram-negative bacteria included Staphylococcus aureus and Escherichia coli, respectively. Finally, 40 Candida isolates were obtained from women with vulvovaginal candidiasis, including 34 C. albicans and six C. glabrata isolates (17).
After culturing the bacterial strains in nutrient agar (NA), incubation was performed for 24 hours at 37°C. Following the inoculation of the Candida isolates in Sabouraud dextrose broth (SDB, Merck, Germany), incubation was performed for 24 hours at 35°C. Then, using sterile distilled water, the yeasts were washed three times and the suspension was used to prepare the 1 × 106 CFU/mL concentration (0.5 MF standard). The positive and negative controls included the fluconazole disc for fungi (10 μg/disc; Hi-Media, India) and gentamicin (10 μg/disc) for bacteria, and DMSO, respectively.
3.7.2. Broth Microdilution for MIC Measurements
After adding the yeast inoculum (100 µl, 106 cells/mL) on SDA (Merck, Germany) and bacterial inoculum (100 µl, 106 cells/mL) on Mueller-Hinton agar (MHA, Merck, Germany), a borer was used to punch six 7-mm wells in the SDA medium; then, 100 μL of the extract dilutions was added, along with DMSO 100% as the negative control (16). The zone of inhibition was measured after incubating the plates at 35°C for 24 hours to determine the anti-Candida activity. The experiments were repeated twice.
3.8. GC-MS Analysis
A GC-MS system (Agilent) with a VF-5 ms silica capillary column (DB-35 ms, 30 m × 250 µm × 0.15 µm) was used for the GC-MS analysis (18), using helium gas (99.99%) as the carrier at a constant flow of 1 mL/min; the temperature of the injector and transfer line was 200°C and 250°C, respectively. On the other hand, the oven temperature increased from 60°C to 280°C at 10°C/min. After maintaining the isothermal temperature for 60 seconds, it increased to 280°C at 10°C/min. In the next step, the sample (1 µL) was injected in the splitless mode (50 - 600 amu; split ratio, 1:10). The total running time was 57 minutes. The retention indices, as well as fragmentation patterns, were compared with the computer library data and literature to identify the extract components. Using the Willey library, the recognized constituents were matched.
3.9. Statistical Analysis
One-way ANOVA was applied at 95% confidence level in SPSS version 16. In addition, the correlation of antioxidant activity with TPC and TFC was determined via linear regression analysis in Excel 2003.
4. Results
4.1. Extraction Yield, TPC, and TFC
The extraction method, growth, and climatic conditions, as well as genetic factors, influence TPC, TFC, and antiradical activity. However, according to the literature (18), the use of a different solvent system, i.e., ethyl acetate: methanol: distilled water, is preferable for the extraction of antioxidant constituents. The percentage of hydroalcoholic extraction yield of C. macropodum leaf extract was 1.86 w/w%.
4.2. Antioxidant Potential
The high antioxidant potential was confirmed on DRSA, reducing power assay, and BCBT.
4.2.1. DRSA
In this primary assay, odd electrons of DPPH are quenched by the extract to decrease absorbance at 517 nm. The leaf extract of C. macropodum showed high antiradical activity in comparison with the standard BHT with the highest inhibition at 1000 μg/mL (14.47) (Table 1).
| Test Compound | IC50 | Inhibition (%) | β-Carotene Bleaching (RAA%) | Reducing Power (%) |
|---|---|---|---|---|
| C. macropodum | 27.63 | 6.38 | 37.61 ± 0.92 | 94.48 ± 3.75 |
| BHT | 14.55 | 14.47 | 100 |
4.2.2. Reducing Power Activity
Compared to vitamin C, the reducing potential of the hydroalcoholic extract was not dose-dependent. The reducing power percentage is demonstrated in Table 1. The leaf extract of C. macropodum showed maximum reducing power (P ≤ 0.05). Analysis of the association between the reducing power activity and phenolic content indicated an R2 of 0.89, while its correlation with the flavonoid content was estimated at R2 = 0.88. Based on these values, phenolic compounds may have greater involvement in the reducing activity, compared to flavonoid compounds.
4.2.3. BCBT
The intensity of yellow color will reduce following the reaction between β-carotene and radicals produced via linoleic acid oxidation. The RAA of C. macropodum extract was measured as follows:
RAA = Asample/ABHT
where ABHT represents the BHT absorbance (standard) and Asample denotes the absorbance of C. macropodum. The calculated RAAs for C. macropodum leaf extract and BHT are presented in Table 1.
4.3. Bioactive Content
4.3.1. TPC Measurements
For the spectrophotometric measurement of TPC (expressed as GAE), the Folin-Ciocalteu method was applied and the standard curve equation was determined (Figure 1):
Absorbance = 0.038× (μg GA) - 0.0132 (R2 = 0.9859)
TPC was calculated by adding the absorbance value in this equation. As shown in Table 2, TPC of C. macropodum leaf extract was 57.43 ± 0.25.
4.3.2. TFC Measurements
For the spectrophotometric measurement of TFC, the Folin-Ciocalteu method was applied and the standard curve equation was measured (Figure 2):
Absorbance = 0.0005 × (μg quercetin) - 0.014 (R2 = 0.9615)
TFC was calculated after entering the absorbance value in the equation. The mean TFC was 138 ± 19.80 mg/g (Table 2).
4.4. Anti-Candida Activity
The anti-C. albicans (n = 34) and anti-C. glabrata (n = 6) activities of hydroalcoholic extracts were confirmed in our analyses (Table 3).
| Variable | Inhibition Zone | MIC | Gentamicin | Fluconazole |
|---|---|---|---|---|
| Candida albicans | 9 | 300 μg/μL | - | 20 |
| Candida glabrata | 8 | 400 μg/μL | - | - |
| Escherichia coli | 7 | 400 μg/μL | 20 | - |
| Staphylococcus aureus | 10 | 300 μg/μL | 21 | - |
4.5. GC-MS Analysis
The presence of phytocomponents was confirmed based on the GC-MS analysis. 15 compounds were detected in C. macropodum (Table 4).
| No | Components | Peak Area, % | RT |
|---|---|---|---|
| 1 | Pentenylamine | 0.058 | 5.308 |
| 2 | Butanoic acid (active valeric acid) | 0.070 | 8.704 |
| 3 | Silanol (formic acid) | 0.160 | 11.216 |
| 4 | 4-vinyl-2-methoxy-phenol | 0.129 | 22.162 |
| 5 | Thiourea | 0.108 | 28.715 |
| 6 | Neophytadiene | 6.524 | 29.205 |
| 7 | Hexadecanoicacid (palmitic acid) | 27.527 | 35.866 |
| 8 | Octadecatrienoic acid (linolenic acid) | 20.296 | 36.175 |
| 9 | Ethylilnoleolate | 6.551 | 36.245 |
| 10 | Octadecadienoic acid linoleic acid | 12.299 | 39.378 |
| 11 | Trimethyloctanal | 6.588 | 42.662 |
| 12 | Pyridinecarboxaldehyde | 4.102 | 44.343 |
| 13 | Benzothiazole | 4.333 | 44.381 |
| 14 | 9-Octadecenoic acid (oleic acid) | 3.522 | 45.765 |
| 15 | Nonacosane | 7.728 | 47.165 |
| Total | 99.99 |
Abbreviation: RT, retention time.
5. Discussion
Recently, the use of medicinal plants has attracted the researchers’ attention. Polyphenols are secondary metabolites, scavenging and quenching free radicals. Coruh et al. (4) showed that TPC of C. macropodum was 34 μg GA/mg. Ebrahimabadi et al. (19) reported that TPC was 29.3 and 30.2 μg GA/mg in the methanolic leaf and flower extracts of C. macropodum, respectively. According to a study by Alavi et al. (20), TPC of the dried extract of C. macropodum was 1.34 μg/mg. In our study, TPC was 57.43 μg GA/mg in the hydroalcoholic leaf extracts of C. macropodum, which is higher than the level reported in the mentioned studies.
On the other hand, 15 compounds were identified in the hydroalcoholic leaf extracts of C. macropodum in this research. The major chemical constituents included palmitic acid, linolenic acid, linoleic acid, nonacosane, trimethyloctane, ethylilnoleolate, neophytadiene, benzothiazole, pyridinecarboxaldehyde, and oleic acid. Based on the findings, omega-3, -6, and -9 and palmitic acid were respectively unsaturated and saturated fatty acids. Unsaturated fatty acids showed higher concentrations than saturated fatty acids. The GC-MS analysis showed that fatty acids were dominant, which is in agreement with a study by Shafaghat (21). Different results regarding the amount of various fatty acids and chemical components may be related to various factors, including season, period of growth, geographical location, altitude, and methods.
Youssef and Noaman (22) showed which benzothiazole derivatives have antibacterial and antifungal activities. Keri et al. (23) conducted a comprehensive systematic review of benzothiazole-based constituents and examined antifungal, antibacterial, antitubercular, antileishmanial, antimalarial, and other medicinal agents. The researchers showed that neophytadiene, a diterpenoid hydrocarbon that belongs to the group of phytanes, has antioxidant and antimicrobial activities (24-26). In the methanolic extract of Apamarga kshara, neophytadiene was a superior TvCK inhibitor compared to other identified compounds (27).
Tao et al. (28) showed that octanal derivatives exhibited strong antifungal activity against Penicillium species. In addition, anti-Candida and antibacterial activities of some Chaerophyllum species showed moderate inhibitory effects (28-30). Ebrahimisadr et al. (31) reported that the extracts of C. macropodum have high antileishmania activities. Our results showed that hydroalcoholic leaf extract of C. macropodum (nearly 17.3% of the total chemical compound) has an inhibitory activity against bacteria and Candida, which is in agreement with the mentioned research and a study by Durmaz et al. (3). It may be due to the presence of compounds, such as benzothiazole, neophytadiene, and octanal, isolated from the hydroalcoholic extract. Therefore, C. macropodum is a candidate for the treatment of some diseases and is used in food industries, as well.
On the other hand, the TPC results showed about 57.43 μg GA/mg in hydroalcoholic C. macropodum leaf extract, which is higher than the amount reported in other studies (4, 19, 20). Differences in the chemical compounds, content, and therapeutic outcomes related to antifungal and antibacterial activities may be due to various factors, such as season, extraction method, growth period, geographic location, and altitude. There may be a new Chaerophyllum species in our region (Rodbarak, Semnan, Iran), based on the plant ecotype theories. Therefore, we will conduct further research on DNA barcoding.