1. Background
The most prevalent oral infectious diseases, such as dental caries, periodontal inflammations, and gingivitis are produced by dental biofilm formation in the oral cavity (1). Dental plaque is a multifaceted biofilm that builds up on the surface of teeth, including more than 500 bacterial species. Moreover, it has recently been shown that oral microorganisms can cause other severe or chronic infectious diseases (2, 3). The value of mouth and teeth hygiene has been known from ancient times until now. It has been documented that Dioscorides, Greek physician, prepared a mouth wash mixture from the extract of plants, milk, oil or vinegar (4). Mouthwashes are oral solutions or liquids used to rinse the mouth in order to remove bacteria, to act as an astringent, to deodorize oral cavity, and for their therapeutic effect by relieving infection or preventing dental caries so they are considered as one of the most effective and safe delivery systems to decrease oral microbes (2, 5). Chlorhexidin is a golden chemical antiplaque, however, it may cause side effects in long usage (6). To overcome the side effects of artificial chemical drugs, it is recommended to search for naturally occurring substances, such as plant extracts, which offer well-tolerated, delicate, and low cost drugs with lower side effects (6, 7). Various herbal extracts such as clove oil, green tea, Aloe vera, Punica granatum, and white oak bark have shown therapeutic effects in the oral cavity when used as mouthwashes (4, 7).
Quercus brantii from the Fagaceae family has many medical and traditional uses. It has shown antibacterial and anti-inflammatory activities. Tannin content of oak husk (Jaft) showed astringent properties and it is used traditionally for treatment of hemorrhoid, diarrhea, gastric ulcer, and inflammations (8, 9).
Zataria multiflora, locally named in Persian as Avishan-e-Shirazi, belongs to the Lamiaceae family. Essential oil of Zataria multiflora has shown anti-bacterial and antifungal effects (10). The most important compounds of essential oil of Zataria multiflora are thymol, carvacrol and paracimol (11, 12).
2. Objectives
In this study a stable formulation containing both oak husk extract and Zataria multiflora essential oil was designed and evaluated and according to the author’s knowledge, this is the first report on such formulation. Preparing a formulation containing tannins from oak husk as astringent agents and the essential oil of Zataria multiflora with antibacterial activity, will introduce a combination, which is more effective and comfortable to administer in patients.
3. Methods
3.1. Material
Propylene glycol (PG), poly ethylene glycol 400 (PEG400), glycerin, and ethanol were purchased from Merck (Germany). Dried oak husk of Quercus brantii and Zataria multiflora were bought from a local store in Shiraz, Fars province, Iran. The plant samples were authenticated by an expert botanist and voucher specimens were preserved with the code PM 712 (Zataria multiflora) and PM 713 (Quercus brantii) at the herbarium of the faculty of pharmacy, Shiraz University of Medical Sciences.
3.2. Oak Husk Dried Extract
The Oak husk of Quercus brantii was powdered mechanically and 200 g of the 180 mesh powder was soaked in deionized water at 80°C to 90°C on the heater for about 10 minutes in order to infuse. The volume was adjusted to 1000 mL by distilled water. It was shaken for one hour in order to complete extraction. The extract was filtered and then freeze-dried.
3.3. Essential Oil Extraction
The essential oil was obtained by hydro-distillation of the fresh powdered leaves of Zataria multiflora, using a Clevenger-type apparatus for about four hour. The essential oil was stored in the freezer until use.
3.4. Tannin Colorimetric Assay
Tannin colorimetric assay was performed by the Follin-Denis method (13). Tannic acid was used as standard. To prepare different dilutions of standard tannic acid, 0 to 10 mL of tannic acid (0.1 mg/mL) was added to separate 100-mL volumetric flasks and then each volume was adjusted to 100 mL by 5 mL of Follin-Denis reagent, 10 mL of sodium carbonate solution (35% w/v) (14), and distilled water. Then, each sample was mixed and kept in the dark for 30 minutes.
3.5. Tannin Calibration Curve Validation
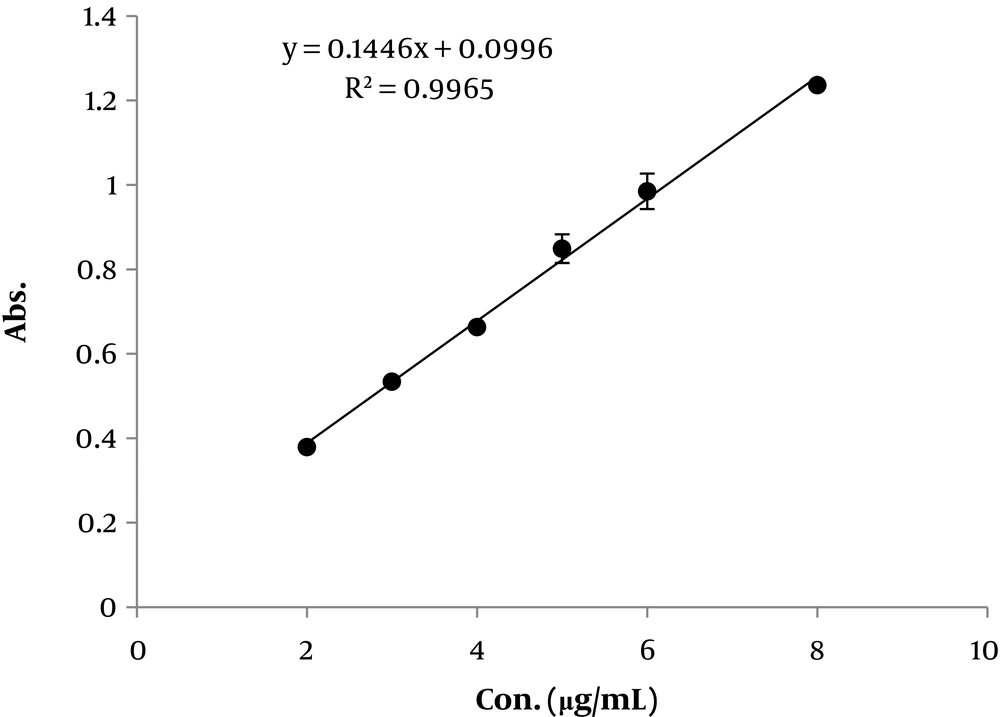
Different concentrations of standard tannic acid (8, 6, 5, 4, 3, and 2 µg/mL) was prepared from stock solution using a serial dilution. Absorbance levels were measured by ultra violet/visible (UV) spectrophotometer (Agilent, USA) at 760 nm. All concentrations were prepared on three different days. Each concentration was examined in triplicates to find the inter-day and intra-day variation. The mean data was used to prepare a calibration curve. Linearity, inter-day, and intra-day precision and accuracy were determined for the calibration curve.
3.6. Essential Oil Analysis
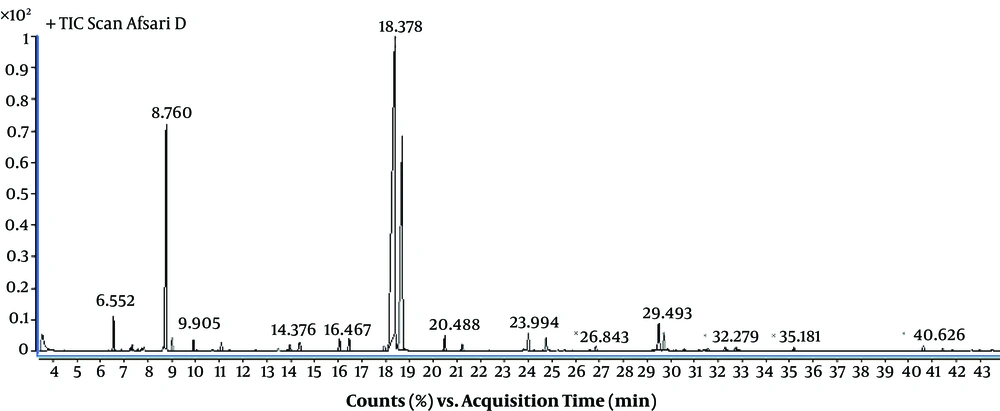
Gas chromatography-mass spectroscopy (GC/MS) analyses of the essential oil were carried out by an Agilent 7890A series gas chromatograph interfaced with an Agilent 7000 mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) in electron impact mode with an ionizing voltage of 70 eV. A silica DB1 column (30 m, 0.32 mm diameter, 0.25-mm film thickness) was used. The flow rate of carrier gas (helium) was 1.2 mL/minute. The injector temperature was 250°C. The oven temperature was increased at a rate of 3°C per minute from 60°C to 280°C and finally maintained for four minutes. The volatile compounds were identified by comparison of their retention indices, referring to compounds known from the literature database, and also by comparing their mass spectra fragmentation with the Wiley Library 7 and NIST data base (15). The yield of essential oil was determined.
3.7. Selection of Mouthwash Base
Different mixture of solvents shown in Table 1 were made as the base for formulations of mouthwash, according to previous studies (not published). To select a suitable base for the mouthwash, different bases with different percentages were examined (16). The formulations were tested for different parameters, such as transmittance percentage, turbidity state, and phase state.
| PG | PEG400 | Glycerin | Ethanol | Water | |
|---|---|---|---|---|---|
| B1 | - | 30 | 30 | 10 | 30 |
| B2 | - | 40 | 45 | 5 | 10 |
| B3 | - | 45 | 50 | 5 | - |
| B4 | 30 | 30 | 30 | 10 | - |
| B5 | 45 | 45 | - | 10 | - |
| B6 | - | 30 | 30 | - | 40 |
| B7 | 50 | 50 | - | - | - |
aValues are expressed as percentage.
3.8. Preparation of Mouthwash Formulations
Five different formulations (F1-F5) with different tannin concentrations (0.1%, 0.2%, 0.5%, 1% and 2%) were prepared by adding 033, 066, 1.3, 2.6, and 5.3 g of dried extract of oak husk. Essential oil was added at fixed amount of 0.15% v/v (equal to 0.064% thymol) to all formulations, according to reported antiplaque concentration of thymol for bacterial enzymes suppression (15). The final volume was adjusted to 100 mL by selected base for better hemogenation. Samples were sonicated for 10 minutes using a bath sonicator.
3.9. Quality Control Tests for Selected Formulations
Quality control tests, including mouthwash pH, tannin content percentage, and essential oil yield were done on days 0 and 45, after preparation of formulations (15).
3.10. Anti-Microbial Activity of Formulations
Microorganisms, including Staphylococcus aureus ATCC 29737, Streptococcus mutans ATCC 35668, Lactobacillus acidophilus ATCC 4356, and Candida albicans ATCC 10231 were obtained from Iran collection of yeast and bacteria center. Fresh cultures of microorganisms were grown in their appropriate media and then microbial suspensions with 0.5 Mcfarland concentrations were prepared in Mueller-Hinton broth for bacteria and sabouraud dextrose broth for C. albicans. Then 50 µL was added to each well in a 96-well microplate, separately, that contained 50 µL of broth media and 50 µL of each formulation (F2, F3) with different dilutions (1, 1:10 and 1:100). The microplate was incubated at 37°C for 18 hours. The microplate was placed in the incubator at 37°C for 18 hours. Finally, the turbidity of the sample and blank (contains 0.5 Mcfarland concentration of microbes) were measured at 600 nm by the Elisa-reader. Then, viability percentage of microbes was found by the below formula (17). Moreover, Persica mouthwash was used as the positive control.
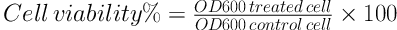
3.11. Statistical Analysis
Statistical comparisons were done by a one-way analysis of variance (ANOVA) followed by Tukey’s test. Differences were considered significant when P < 0.05.
4. Results
4.1. Tannin Colorimetric Assay
As mentioned in the methods, the colorimetric assay was used for tannin determination. Calibration curve validation is shown in Figure 1. The calibration curve equation and the linearity was as follows, absorbance = 0.1446 (concentration) + 0.0996 and 0.9965, respectively. Results indicated an average of 97.1 ± 1.63% and 98.8 ± 0.56 precision for all concentrations inter-daily and intra-daily. The average accuracy for all concentrations was 98.7 ± 1.2%.
4.2. Tannin Determination in the Extract
The tannin concentration of extracted oak husk was determined using the analysis method and it was found to be 33.7 ± 4.3% of dry powdered extract (≈ equivalent to 337 mg/g dry extract).
4.3. Essential Oil Analysis
Identification of different components of Zataria multiflora volatile oil was done and their percentage was determined using their retention indices. The main constituents of Z. multiflora used in this study, were Thymol (44.23%), carvacrol (23.8%) and cymene (13.02%). The essential oil components were shown in Table 2.
| Compound | RT | KI | Percentage |
|---|---|---|---|
| α-pinene | 6.552 | 938.3 | 1.54 |
| Cymene | 8.742 | 1018.4 | 13.02 |
| 1,8-cineole | 9.013 | 1027.2 | 0.8 |
| γ-Terpinene | 9.905 | 1054.4 | 0.58 |
| Linalool | 11.077 | 1086.6 | 0.53 |
| 4-Terpineol | 13.961 | 1165.3 | 0.53 |
| α-Terpineol | 14.367 | 1175.4 | 0.83 |
| Thymyl methyl ether | 16.053 | 1217.5 | 0.70 |
| Carvacrol methyl ether | 16.467 | 1228.3 | 0.87 |
| Unknown | 17.928 | 1264.3 | 0.62 |
| Thymol | 18.342 | 1274.06 | 44.23 |
| Carvacrol | 18.694 | 1282.1 | 23.8 |
| Thymol acetate | 20.488 | 1326.4 | 1.03 |
| Durenol | 21.218 | 1345.2 | 0.47 |
| Trans-caryophyllene | 23.994 | 1413.8 | 1.18 |
| Valencene | 24.761 | 1434.8 | 0.89 |
| Ledene | 26.834 | 1488.5 | 0.37 |
| Unknown | 29.493 | 1559.2 | 2.09 |
| Longifolenaldehyde | 29.700 | 1564.6 | 1.38 |
| Unknown | 30.575 | 1586.9 | 0.12 |
| Unknown | 31.566 | 1613.9 | 0.21 |
| Unknown | 32.288 | 1635.1 | 0.26 |
| Unknown | 32.738 | 1648.1 | 0.36 |
| α-cyperone | 35.172 | 1717.4 | 0.22 |
| Unknown | 40.617 | 1822.3 | 0.36 |
| Unknown | 41.455 | 1908.5 | 0.21 |
| Total identified | 92.97 |
The chromatogram of GC analysis of Z. multiflora is shown in Figure 2.
4.4. Preparation of Mouthwash Formulations
As shown in Table 3, transmittance percentage, turbidity state, and phase state were reported for all formulations. Considering the absence of ethanol in the formulation, B7 was selected as the best base for preparing formulations. Final formulation ingredients are reported in Table 4.
| Base | Transmittance, % | Turbidity State | Phase State | Ethanol Presence |
|---|---|---|---|---|
| B1 | 89.6 | A little opace | One-phase | + |
| B2 | 24.9 | Opace | One-phase | + |
| B3 | 91.2 | Clear | Two-phase | + |
| B4 | 98.8 | Clear | One-phase | + |
| B5 | 99.8 | Clear | One-phase | + |
| B6 | 47.9 | Opace | Two-phase | - |
| B7 | 96.1 | Clear | One-phase | - |
| Formulation | Tannin | Essential Oil | Base |
|---|---|---|---|
| F1 | 0.1 | 0.15 | P.G:PEG400 (1:1) |
| F2 | 0.2 | 0.15 | P.G:PEG400 (1:1) |
| F3 | 0.5 | 0.15 | P.G:PEG400 (1:1) |
aValues are expressed as percentage.
4.5. Quality Control Tests for Selected Formulations
The formulations containing 1% and 2% tannin were excluded from the study because of precipitation after a few days. For other formulations, pH, tannin content, and essential oil yield were evaluated. pH and tannin content results are reported in Table 5. The yield percentage of the essential oil obtained from formulation F2 on day 0 and 45 was 90% and 86.6%, respectively.
| pH | Tannin, % | |||
|---|---|---|---|---|
| Day-0 | Day-45 | Day-0 | Day-45 | |
| F1 | 5.3 ± 0.1 | 5.1 ± 0.2 | 101.4 ± 2.6 | 100.7 ± 3 |
| F2 | 5.2 ± 0.2 | 5.1 ± 0.1 | 98.6 ± 1.3 | 97 ± 1.7 |
| F3 | 5.5 ± 0.2 | 5.2 ± 0.1 | 100.9 ± 1.4 | 99 ± 1.6 |
4.6. Anti-Microbial Activity of Formulations
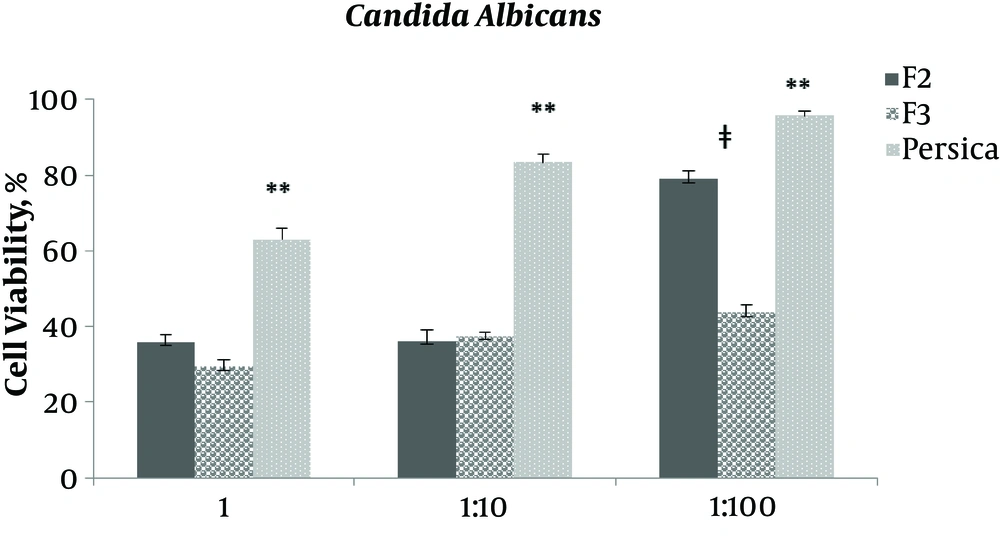
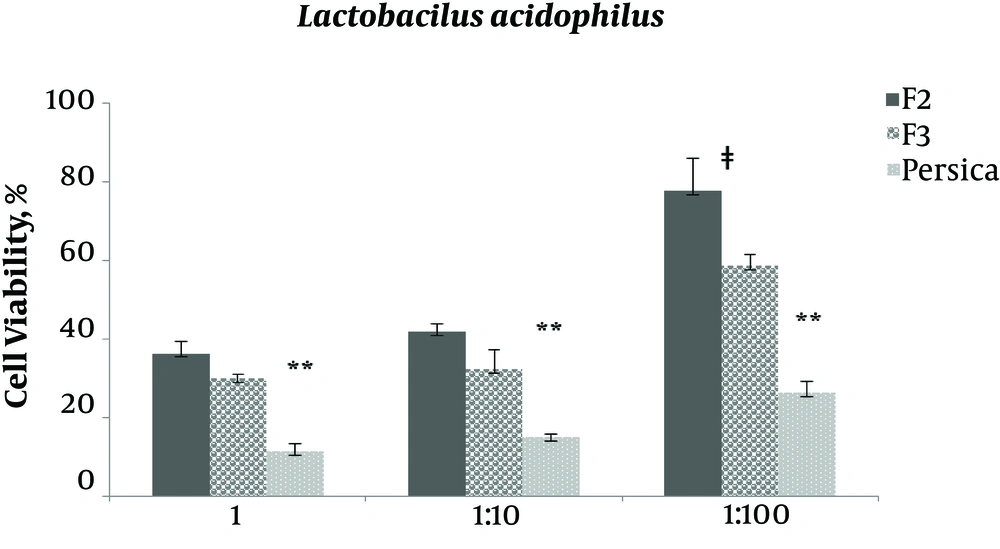
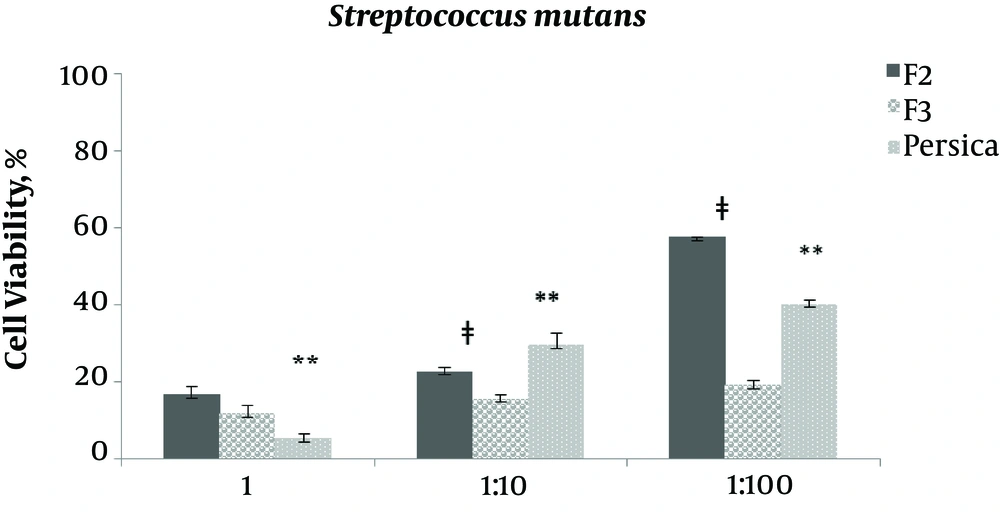
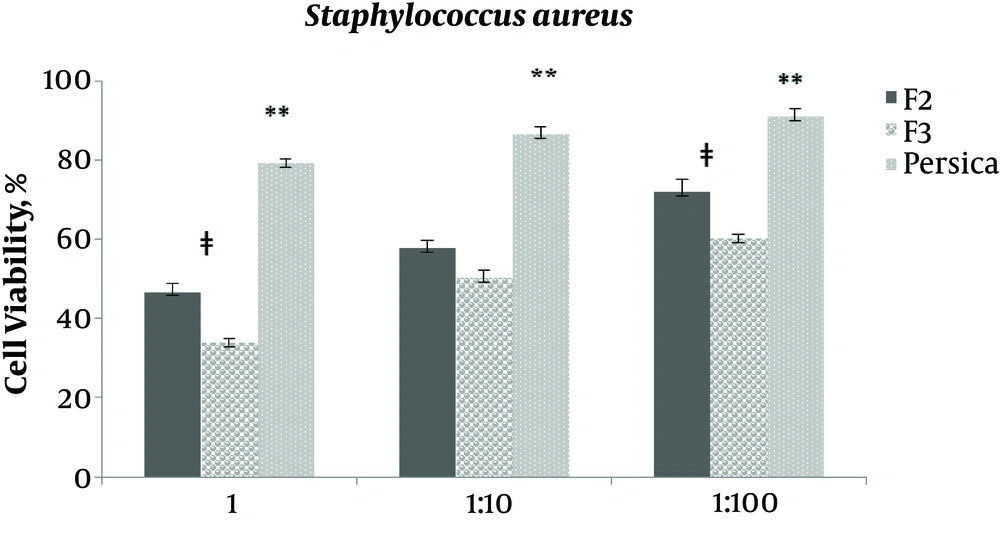
As indicated in Figures 3 - 7, viability percentage of four different microorganisms in presence of selected formulations compared with the control was calculated. According to usual mouthwashes dilution at the time of consumption, the antibacterial efficacy of two final selected formulations was examined at three different concentration (not diluted, 1:0 and 1:100 diluted). Formulation F1 was not entered in this test due to rejection in limit test experiments (data not shown).
5. Discussion
Oral hygiene is an important aspect of periodontal health. There is a balance in a person’s oral microbial population. It is necessary to maintain this balance to prevent opportunistic microorganism proliferation (15). An optimum mouthwash has some advantages, such as having antiseptic effects on the mouth, washing the food residue on the gingival (gum) medium and teeth, reducing the mouth bacteria, masking and neutralizing halitosis, and introducing a good taste and sense of freshness in the mouth.
It has been shown that herbal mouthwashes do not cause change in the color of teeth or unpleasant taste. Reducing the plaque accumulation and gingival inflammation are other advantage of herbal mouthwashes in comparison to chlorhexidine mouthwash. Because of their minimum side effects, herbal mouth washes can be recommended for long term use (18). On the other hand, presence of ethanol in commercial mouthwashes seems to be problematic due to different reported side effects, such as xerostomia and oral and pharyngeal cancer induction (19), thus, elimination of ethanol may be a good strategy for mouthwash formulation. Therefore, the ultimate goal of the present study was to prepare a mouthwash with two beneficial natural products (Persian oak tannins and Zataria multiflora essential oil) in absence of ethanol.
Tannins are a group of polyphenols that precipitate microbial proteins and prevent the development of microorganisms. Polyphenols and tannins are the main substances in oak tree and are abundant in all parts of Quercus brantii. Tannins are biosynthetic materials, which have a potent antibacterial effect (13). Aslani et al. showed that the seed hull (Jaft) of Q. brantii contains considerable polyphenols (9). Ebrahimi et al. reported 162 to 648 mg/g of tannic acid/g dry extract for 27 populations of Quercus brantii, however, the current results indicated 340 mg tannic acid /g dry extract, which is within the reported range (20).
Furthermore, Z. multiflora, is used as an important herbal medicine in Iranian traditional medicine for 1000 years to cure stomachache and agitation. It has several biological and pharmacological properties. There are many studies that have shown that Z. multiflora has antibacterial, antifungal, and antioxidant activities (21).
Antimicrobial activity of mouthwash solutions is important to ensure their efficacy in eliminating harmful periodontal bacteria, which leads to prevention of future dental carries, gingivitis, and periodontitis (22). The most common causative dental plaque organisms, Candida albicans, Lactobacillus acidophilus, Streptococcus mutans, Staphylococcus aureus (22), were examined to evaluate selected mouthwashes.
According to the ANOVA test for Candida albicans F2 and F3, efficacy was similar for not diluted and 1:10 diluted (P > 0.05) solutions while in 1:100 dilution F3 was significantly (P = 0.000) more efficient than F2. It is interesting that at all concentrations, F2 and F3, even 1:10 and 1:100 diluted formulations, were significantly (P = 0.000) more efficient than Persica mouthwash.
For Lactobacillus acidophilus F2 and F3, efficacy was similar for not diluted and 1:10 diluted (P > 0.05) solutions, while at the 1:100 dilution, F3 was significantly (P = 0.011) more efficient than F2. In not diluted, 1:10, and 1:100 diluted concentrations, Persica mouthwash was significantly (P < 0.05) more efficient than F2 and F3; however, F3 was more efficient than F2. It should be noted that Persica mouthwash was not diluted.
For Staphylococcus aureus, F2 and F3 efficacy was similar for 1:10 diluted (P > 0.05), while in not diluted and 1:100 dilution, F3 was significantly (P = 0.002 and P = 0.003) more efficient than F2. It is interesting that at all concentrations, F2 and F3, even 1:10 and 1:100 diluted formulations, were significantly (P < 0.05) more efficient than Persica mouthwash.
For Streptococcus mutans, F2 and F3 efficacy was similar for not diluted (P > 0.05) while in 1:10 and 1:100 dilution F3 was significantly (P = 0.015 and P = 0.000) more efficient than F2. In not diluted concentration, F3 had similar efficiency as Percia mouthwash (P > 0.05).
Results showed that the selected formulations of F2 and F3 (containing 0.2% and 0.5% tannin) had better antimicrobial activity against all examined microorganisms, except Lactobacillus acidophilus, compared with Persica.
F2 and F3 in not diluted and 1:10 diluted concentration had the same effect against Candida albicans, while F3 was significantly more effective than F2 in 1:100 diluted form, therefore, F3 was more affordable. This antibacterial effect pattern was similar for Lactobacillus acidophilus while it should be noted that the potency was less than Persica.
The best antibacterial activity against S. aureus, belonged to F3 with 0.5% tannin. Persica mouthwash (in not diluted concentration) was significantly more effective than the selected formulations against S. mutans. However, F2 and F3 had the same activity and significantly more efficacy than Persica mouthwash in 1:10 diluted concentration. In 1:100 diluted concentration, F2 was significantly more effective than Persica mouthwash and F3.
5.1. Conclusion
In this study, the mouthwash formulations, F2 and F3, which contained 0.2% and 0.5% tannin, were the best formulations with adequate stability. The results also showed anti-microbial activity in both mouthwash formulations. Therefore, these two preparations can be used for further studies to establish their efficacy and safety as anti-bacterial, anti-hemorrhage, and freshener herbal mouthwashes.