1. Context
Nanotechnology provides many components used in various industries in many developed countries (1). This new technology uses different compounds and aims to design materials in nanometer dimensions and better function (2). Although nanotechnology has prepared some new manufacturing opportunities in various industries, unfortunately, the properties of many of the used substances have not yet been completely determined; in addition, their toxicity levels have not been evaluated for humans in many cases (3). The suitable properties of metal nanoparticles (NPs) (such as being small and large sizes) and versatile surface features (such as charge, conductivity, shape, melting, and freezing point difference) have led metal NPs and metal oxide NPs to be used in various industries. One example is zirconium (Zr), which itself and its derivative are widely used. Some previous studies have revealed that NPs can penetrate through biological barriers, accumulate in different organs, and induce toxic effects, such as cell death, DNA damage, oxidative stress, and morphological changes; therefore, cytotoxicity and genotoxicity assessment of NPs is necessary (4-6). Zirconium and its derivatives [including zirconium oxide (ZrO2)] have been used to produce some useful NPs in many fields related to human health (including dental implants, bone joint replacements, and drug delivery vehicles) (7).
Ag@ZrO2 nanoparticles, consisting of a ZrO2 core coated with Ag nanoparticles, exhibit efficacy against various Gram-negative bacteria such as Escherichia coli and Staphylococcus aureus, as well as certain fungal species like Candida albicans, Candida glabrata, Aspergillus niger, and Aspergillus flavus.
Thus, it was suggested to be used as a microbiocide in water treatment (4). Although some studies have investigated the positive effect of Zr and its derivatives and suggested the use of Zr NPs in various industries, some other studies have evaluated and proven the cytotoxicity and genotoxicity effects of these compounds (8). It has been shown that ZrO2 NPs induce toxic mechanisms in macrophages by perturbation in the cytoskeleton and phagocytosis and increasing methylglyoxal-associated DNA damage (5). The other recent study supported that ZrO2 NPs induce DNA damage and apoptosis in mouse fibroblast cell lines (3). Further, it has been indicated that exposure to ZrO2 causes toxicity in zebrafish embryos, leading to mortality, hatching delay, and malformation in aquatic environments (8). Novel colloidal silica spheres coated with crystalline or amorphous Zr (SiO2@ZrO2cryst or SiO2@ZrO2am) have been reported to induce both cytotoxicity and genotoxicity in a human osteosarcoma cell line (MG-63) by reducing cell viability and increasing reactive oxygen species (ROS) and DNA damage at 5 and 25 μg/mL (9). According to the importance of the use of Zr in biomedical fields, it is important to review studies that have investigated the genotoxicity and cytotoxicity effects of Zr from different aspects and in different contexts (10). Also, the synthesis of Zr NPs in a green way has recently attracted the attention of researchers (Table 1). The synthesis of Zr NPs is usually done by physical and chemical methods. Physical methods have high costs, and chemical methods cause a lot of pollution to the environment in terms of toxicity. For this reason, producing NPs in a green way can reduce the pollution related to zirconium synthesis (11-15). This study reviewed, compared, and discussed the results of different studies in the mentioned field.
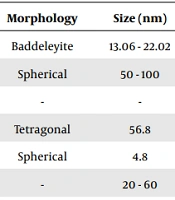
| No. | Plant Origin | Morphology | Size (nm) | Part | Application | Reference |
|---|---|---|---|---|---|---|
| 1 | Capscicum annuum | Baddeleyite | 13.06 - 22.02 | Leaves | Antibacterial, antifungal | (16) |
| 2 | Aloe vera | Spherical | 50 - 100 | Leaves | Antimicrobial, antifungal | (17) |
| 3 | Nyctanthes arbor-tristis | - | - | Flowers | Antibacterial | (18) |
| 4 | Lagerstroemia speciosa | Tetragonal | 56.8 | Leaves | Cytotoxicity study, photocatalytic activities | (19) |
| 5 | Sargassum wightii | Spherical | 4.8 | - | Antibacterial | (20) |
| 6 | Punica granatum | - | 20 - 60 | Fruit peels | Antibacterial | (21) |
| 7 | Helianthus annuus | Monoclinic | 40 | Seeds | Biocompatible in medical implantation, antibacterial | (22) |
| 8 | Eucalyptus globulus | Spherical | 9 - 11 | Leaves | Antioxidant, cytotoxicity | (23) |
| 9 | Aegle marmelos | - | 38 | Fruit | Antibacterial | (24) |
2. In Vitro Studies
2.1. Cell Line Studies for Zr Toxicity
Recently developed Zr nanostructures may have harmful effects on cells in a dose-dependent manner (25, 26). Zirconium can affect osteoblast cells. In the study by Ye and Shi, the toxicity effect of Zr NPs on osteoblast-like 3T3-E1 cells was investigated. Using cytotoxicity tests, they showed that Zr NPs with the production of ROS caused apoptosis, and death could be harmful to osteoblast-like 3T3-E1 cells in a dose-dependent manner (27). Another study showed that Zr might drastically up- or downregulate the expression of a number of genes that control a wide variety of functional activities in osteoblast-like (MG-63) cells, including cell cycle regulation, immunology, signal transduction, and vesicular transport (19).
Dental implants are one of the causes of chronic Zr toxicity. In this regard, several studies have been conducted on the impact of Zr on different cells. These studies have shown that Zr can be poisonous and damage many different types of tissues and cells when exposed for an extended period of time (28, 29). Also, using a Zr toxicity test on periodontal ligament cells with the lentiviral gene transfer technique of human telomerase reverse transcriptase, it was determined that Zr damaged cellular DNA in a dose-dependent manner (30).
Neurotoxicity of Zr NPs was also tested on PC12 and N2a cells from a rat pheochromocytoma and mouse neuroblastoma, respectively. Zirconium nanoparticles significantly decreased cell viability and glutathione (GSH) levels in PC12 and N2a cells while significantly increasing intracellular ROS and MDA levels. Zirconium nanoparticles have cytotoxic and genotoxic effects on PC12 and N2a cells in time- and concentration-dependent manners at concentrations higher than 31 g/mL (31).
Zirconium can have destructive effects on blood cells, as mentioned in previous studies. For example, a study conducted on Zr revealed a considerable DNA damage induction at 5mM and apoptosis in human (Jurkat) T cells at concentrations over 0.5 mM (32); in addition, tumor necrosis factor α (TNF-α) release and macrophage death in the J774 mouse cell line were both induced by Zr particles of around 0.6 μm (33). The tumor suppressor proteins p53 and p73 are produced in greater amounts when human bone marrow-derived stromal stem cells (hMSCs) are exposed to submicron particles of Zr (34). Sai Saraswathi and Santhakumar evaluated the cytotoxicity of ZrO2 NPs and the aqueous extract of Lagerstroemia speciosa on MCF-7 cell lines. The results showed that this compound containing Zr NPs was able to inhibit cell growth on the MCF-7 cancer cell line in a dose-dependent manner compared to the control group (19). This cytotoxic property of Zr may have an inhibitory effect on the uncontrolled growth of cancer cells and can be used in anticancer compounds and drugs.
3. In Vivo Studies
3.1. Non-animal Studies
3.1.1. Microbial Toxicity of Zr
The rise of antibiotic resistance has emerged as a significant global concern. Today, with the advancement of nanobiotechnology knowledge, Zr NPs can be used to prevent infections in implants and antimicrobial drug delivery systems (35, 36). For instance, Imran et al. investigated the antibacterial activity of ZrO2 NPs doped with Fe3O4, showing an increase in antibacterial activity against S. aureus, E. coli, and Bacillus subtilis (37). Similarly, Rad Goudarzi et al. investigated the effect of ZrO2 NPs doped with Fe3O4 and hydroxyapatite on S. aureus; using microbial tests, they showed that this compound also had significant lethal effects on S. aureus (38). Jangra et al. showed that Zr only had antibacterial properties against E. coli, while in addition to significant antifungal activity, also its various compounds have antibacterial activity against S. aureus as well (39). In the study by Doskocz and Załęska-Radziwiłł, the effect of ZrO2 NPs and their macromolecules was examined (40). They showed that NPs might have a strong killing effect on microorganisms. In fact, the results of this study show that Zr NPs are more toxic than Zr.
3.1.2. Plants
The effects of Zr on plants have also been investigated. Plants absorb Zr in various quantities, primarily through their root system, which enables its entry into the food chain. After uptake from water or soil, Zr tends to accumulate predominantly in root cells. It is worth noting that Zr has no recognized essential function in the metabolism of plants or animals. However, it can still have significant effects on plant growth and influence plant enzyme activity. In response to Zr-induced toxicity, plants have evolved multiple defense mechanisms to cope with these adverse effects. These strategies include sequestering Zr in their root tissues and activating diverse antioxidants to prevent its harmful effects (41). For example, Fodor et al. found that Zr ascorbate caused physiological changes in wheat seedlings. It was found that after 9 days of exposure to different concentrations, this compound inhibited germination, delayed the growth of roots and shoots, and increased the activity of antioxidant enzymes. Also, it was found that Zr ascorbate is only harmful at a concentration of 100mM and above (42). Although there are limited published data on the subject, it appears that the phytotoxicity of Zr is generally low.
3.1.3. Insects
In a study, researchers carried out a genotoxic evaluation of ZrO2 NPs and their microparticulate forms in Drosophila melanogaster using the wing somatic mutation and recombination assay. Third-instar larvae were fed with ZrO2 NPs and their microparticulate forms at concentrations of 0.1 to 10 mM. The results showed that no significant increases were observed in the frequency of all spots, indicating that these NPs cannot induce genotoxic activity in the wing spot assay of D. melanogaster. Negative data were also obtained with the microparticulate forms. This indicates that the nanoparticulated form of ZrO2 does not modify the potential genotoxicity of its microparticulate versions (43).
Also, in an experiment, different Zr(NO3)4nanocomposites were mixed in insect food. This study showed that the composite mixture (10% hBN and 90% ZrO2) had no significant effect on insect growth, had weak cytotoxicity, and might be safe in the field of biomedicine. However, nanocomposites (90% hBN 10% ZrO2) caused more cytotoxicity and phenotypic changes in the insect (44).
3.2. Animal Studies
3.2.1. Fish
In India, Mishra and Mahapatra conducted a study on the function of different organs of Channa punctata (a species of snakehead) in terms of toxicity with heavy metals such as Zr. The results showed that chronic exposure to lethal concentrations of ZrOCl2led to a significant decrease in protein and fat concentrations in their liver and muscle. In addition, degenerative changes were detected in different parts of the fish, including the gill, intestine, stomach, and liver exposed to Zr oxychloride, along with various nuclear abnormalities and micronucleus (45).
P et al. investigated the toxicity of ZrO2 NPs in zebrafish (Danio rerio). They used concentrations of 1, 2, 3, 4, and 5 μg/mL of ZrO2 NPs and finally concluded that this substance caused acute developmental toxicity in these embryos, causing death, hatching delay, and malformations at lower concentrations (46).
3.2.2. Rabbits
Kang et al. reported sensitization to compounds of beryllium, zirconium lactate, zirconium aluminum glycinate, and aluminum in rabbits inoculated with multiple injections of sodium zirconium lactate. The results showed inhibition of macrophage migration and skin reactivity. Histologically, sodium zirconium lactate and aluminum chlorohydrate induced organized foreign body granulomas after intradermal injection in normal and inoculated rabbits. Sodium zirconium lactate and aluminum chlorohydrate induced organized foreign body granulomas after intradermal injection in normal and treated rabbits (47). Furthermore, the immunological response of pulmonary bronchus cells to aluminum chlorohydrate and zirconium salts (sodium, lactate, and aluminum glycine) was investigated in rabbits. As a result, aluminum and zirconium salts can cause respiratory bronchiolitis and activation of alveolar macrophages in a relatively high dose (10 mg) (48).
3.2.3. Rats
Landsiedel et al. conducted a study to evaluate the impact of 13 nanomaterials, including ZrO2, on the lungs of rats. The results indicated that exposure to aerosol concentrations of ZrO2 up to 10 mg/m3 did not elicit any treatment-related effects on cytological levels. Additionally, ZrO2 did not cause alterations in protein, enzyme, or cytokines levels in bronchoalveolar lavage fluid or in lung tissue. Furthermore, blood parameters and acute phase protein levels in the blood remained unchanged throughout the study. There were no histopathological changes in the respiratory tract, and the rate of cell proliferation and apoptotic reactions in lung cells were not significantly different from the control groups (49). In addition, in the study conducted by Spiegl et al., 270 animals from 5 different species were exposed to zirconium compounds through inhalation for 6 hours a day and 5 days a week at the rate of 75 mg/m3. The results showed that ZrO2 did not cause any specific changes in mortality, growth rate, blood non-protein nitrogen or fibrinogen, urinary protein, hematological factors, or structural structure, but Zr at the rate of 6 mg/m3 caused suspicious changes in the concentration of blood compounds. It increased blood hemoglobin and red blood cell counts in dogs and also increased mortality rates in rats and guinea pigs. Also, inhaled zirconium compounds were mainly deposited in the lymph nodes and lungs (50). In addition, it was found that rats that were exposed to ZrO2 particles had a significant increase in the level of liver and kidney enzymes, and this substance also caused increased levels of free radicals in their blood. They used 25-, 50-, and 100-ppm NPs (51). Mehdikhani et al. reported that ZrO2 NPs probably damage the testes by increasing the production of ROS and free radicals. They observed that rats receiving 400 ppm of Zr had a contraction of the seminiferous tubules and decreased lumen space in the testicles compared to normal rats (52). In addition to examining the biodistribution of ZrO2 NPs, GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators examined oxidative stress factors, histopathology, and immunohistochemistry in the spleen, kidney, heart, brain, and lung of rats at 6-time points after a single injection of ZrO2 NPs, finding that Zr can cause continuous oxidative damage and promote/inhibit cell proliferation in these organs. Accordingly, they concluded that Zr can cause destructive effects on these organs (53). Brown et al. found that zirconium lactate and barium zirconate caused poor weight gain, pathological changes consistent with chronic interstitial pneumonitis, very little deposition of fibrous tissue, absence of granuloma, and increased amount of Zr in the lung tissue of rats treated with these substances (54). Furthermore, in a study to evaluate the intrinsic effects of the trace elements zirconium, niobium, antimony, and vanadium (as well as to re-evaluate the effects of lead, 603 rats of the Long-Evans strain were fed a diet containing relatively small amounts of these elements in an environment reasonably free of trace contaminants. An increased incidence of glycosuria was observed in zirconium, niobium, and lead groups (55).
3.2.4. Mice
The effect of Zr on mice has also been determined. Dekkers et al. investigated the effect of Zr doping of CeO2 NPs on the respiratory and cardiovascular system in mice. According to their results, subacute inhalation of CeO2 NPs caused minimal pulmonary and cardiovascular effects within 4 weeks after exposure, and Zr doping has a limited effect on these responses (56). Moreover, in another study, to evaluate the intrinsic effects of certain trace elements, 540 mice were fed a diet of rye, corn oil, and skimmed milk containing moderate amounts of zirconium and niobium and no detectable antimony or fluorine in a relatively free environment. Trace pollutants were given. It was found that Zr toxicity caused minor toxicity in both control and treatment groups. Chromium, fluorine, and nickel had no demonstrable intrinsic toxicity, while tellurium, arsenic, tin, and vanadium showed the greatest toxicity (57).
3.2.5. Mini Pig
He et al. investigated Ti and ZrO2 implants in mini pig maxillae. After 12 weeks of implantation, the content of Ti released from Ti implants was twice more than the content of Zr released from ZrO2 implants in mini pig maxillae. The half maximal effective concentration (EC50) of ZrO2 NPs and ZrO2 microparticles was 13.96 and 80.99 mg/mL, respectively. Also, these substances can induce DNA damage at 70 and 810 µg/mL, respectively (30).
4. Conclusions and Perspectives
Zirconium and its derivatives can have a very effective effect on human health and prevent infections from different sources, but it should be noted that their excessive and long-term use can have negative effects on the environment and living organisms. Exposure to Zr has been reported through various ways, including inhalation, ingestion, skin penetration, etc. Because Zr is lipophilic, it can accumulate in different organs (lungs, liver, spleen, heart, brain, and kidney) and induce oxidative stress. In addition to their small size, receptor-mediated endocytosis and passing through paracellular connections have caused them to pass through the placenta and fetal blood-brain barrier (BBB) and accumulate in the fetal brain. It has been proven that ZrO2 NPs cause substantial DNA damage to human T cells. Furthermore, they can trigger apoptosis and inhibit cell growth in cell lines. However, more studies are needed to investigate the chronic and long-term toxicity of Zr.