1. Background
Methotrexate (MTX), a folic acid analog, is a common and widely used cytotoxic chemotherapeutic drug (1), inhibiting the dihydrofolate reductase (DHFR) enzyme and DNA synthesis (2). Methotrexate has been widely considered for the treatment of malignancies such as osteosarcoma, lymphocytic leukemia, testicular tumors, non-Hodgkin lymphoma, mammary gland tumors, and head and neck cancer (3). Additionally, in different inflammatory diseases, for example, rheumatoid arthritis, psoriasis, polymyositis, and systemic lupus erythematosus, MTX acts as a first-line drug (4).
Unfortunately, the efficacy of MTX has sometimes been limited by severe side effects and toxic sequel of different organs, such as hepatotoxicity, nephrotoxicity, cardiotoxicity, and intestinal toxicity (5). The cytotoxic effect of MTX is not limited to cancer cells; it affects normal tissues that have high proliferative activity, for instance, hematopoietic cells of the bone marrow and the mucous membrane of the alimentary system (6).
Gastrointestinal (GI) mucositis is a well-recognized toxicity associated with some standard-dose chemotherapy regimens usually used with MTX (7). The mouth and small intestine seem to be most affected by mucositis (8, 9). Ciralik et al. postulated that MTX caused damage to the mucosa of the small intestine, leading to nausea, vomiting, diarrhea, stomatitis, decreased absorption, and GI ulceration (10). About 40% of patients receiving MTX at standard doses experience intestinal mucositis, while this rate has been reported as almost 100% at high doses (7).
When intestinal mucositis appeared, the slime layer is exhausted with a reduced level of mucins (11). Malfunction of the mucous layer, in turn, exacerbated inflammatory symptoms and impaired the water-holding capability of the GI tract, caused aquaporins overexpression, and ultimately prompted serious diarrhea in intestinal mucositis (12).
In addition to diarrhea, there are other side effects such as obvious body-weight reduction, decreased nutrient uptake, and restricted ability to tolerate therapy, which resulting in the delay of succeeding cycles or premature drug withdrawal (13).
Despite extensive clinical trials on chemotherapy-induced intestinal mucositis over the years, there are currently no viable treatment options to effectively combat this condition (14).
It is understood from the investigations that reactive oxygen species (ROS) have a role in the intestinal toxicity caused by MTX (15). Also, Kaynar et al. (16) and Gulgun et al. (17) demonstrated that in the small intestine of the treated rats with MTX, there were increased oxidant and decreased antioxidant parameters (16, 17). Another study by Olsen et al. postulated that MTX could up-regulate the expression level of pro-inflammatory cytokines of IL-1, interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF alpha) in the monocytes (18), de Araujo Jr et al. reported that in the small intestine, MTX inhibits the signaling pathway of suppressor of cytokine signaling-1 (SOCS-1), which has a negative regulatory role in the production of pro-inflammatory cytokines (19).
Given the impact of oxidative stress and inflammatory factors on the occurrence of intestinal mucositis, researchers suggest that it may be possible to reduce harmful effects on mucositis by using substances that possess antioxidant and anti-inflammatory properties (20).
Gallic acid (GA; 3,4,5-trihydroxybenzoic acid) is a phenolic compound widely found in gall nut, green tea, hops, grapes, red wine, berries, and oak bark (21).
It is understood from the literature that GA owns several pharmacological properties, including antioxidant, anti-inflammatory, ant mutagenic, and anti-tumor activities (22), and due to these properties, could ameliorate toxicity of different organs (23-25). Another study by Khodayar et al. showed that in a rat model of ulcerative colitis, GA can decrease the levels of inflammatory mediators (26).
2. Objectives
The present study was designed to investigate the protective effect of GA against oxidative stress, pro-inflammatory status, and histopathological injuries of MTX administration-induced intestinal mucositis in the duodenum and jejunum of male Wistar rats.
3. Methods
3.1. Chemicals
Gallic acid was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA, CAT. NO: G7384). Methotrexate was obtained from NanoAlvand Laboratories, Iran. Malondialdehyde kit was purchased from ZellBio Company, Catalog No. RK09070. A superoxide dismutase kit was provided by ZellBio Company, Catalog No. RK07054.
In addition to the glutathione peroxidase kit that was purchased from the same company, Catalog No. RK03696, Eliza kits of interleukin 2 (IL-2) and IL-6 were obtained from Abcam Company (ab221834), (ab222503).
3.2. Animals
This study was performed according to the ethical standards and approved protocols of the Committee of Animal Experimentation of Dezful University of Medical Sciences, Dezful, Iran (code: IR.DUMS.REC.1398.023).
Twenty-eight male Wistar rats (weight: 170 - 190 g, 12 - 14 weeks old) were obtained from the animal house of Dezful University of Medical Sciences, Iran. The animals were maintained in polypropylene cages under a 12-hour light/dark cycle. The temperature was maintained at 25°C and humidity at 60%. Food and water were available ad libitum.
3.3. Experimental Design
The animals were randomly divided into 4 experimental groups; each group consisted of 7 rats. Control group: This group was treated with normal saline (2 mL) by gavage for 10 days and one intra peritoneal (IP) saline injections on the sixth day. Gallic acid group: Received gallic acid 30 mg/kg, orally for 10 days (23); MTX group: Treated with a single dose of MTX (20 mg/kg, IP) (27) on the 6th day; finally, MTX-GA group: Treated with gallic acid (30 mg/kg, gavage) for 10 days and a single dose of MTX (20 mg/kg, IP) on the 6th day. The weight of each animal was measured on the first and last day of the study.
3.4. Sample Collection
After 24 h of the last administration, the rats were sacrificed under deep anesthesia using a combination of ketamine and xylazine (60/6 mg/kg, IP). Blood samples were collected from rats’ left ventricle, and the serum was separated through centrifugation for 10 min at 1000 g and stored at -20°C until analysis. Samples of the proximal part of the duodenum and mid-piece of the jejunum were isolated and washed with cold saline. The tissues were fixed in phosphate-buffered formalin (10% w/v) for histological studies.
3.5. Serum Biochemical Assessments
3.5.1. Serum Malondialdehyde Level Assay
Serum was used to assess lipid peroxidation by the thiobarbituric acid response to the malondialdehyde (MDA) assay kit (ZellBio GmbH, Ulm, Germany). The presence of trichloroacetic acid leads to the production of a red complex. The red color response produced at 532 nm was determined using a nanodrop. This method determines the level of MDA with a sensitivity of 0.1 μM.
Some researchers including Buege and Aust, Rostami et al. and Naserzadeh et al., also used the method of determining serum MDA to determine the state of oxidative stress (28-30).
3.5.2. Serum Nitric Oxide Assay
Nitric oxide concentration was determined using an indirect method based on the measurement of nitrite concentration in serum according to Green et al.’s reaction (31). Nitrite concentrations were determined by spectrophotometric analysis at 540 nm. No products were expressed as nmol/mL.
3.5.3. Serum Superoxide Dismutase (SOD) Activity Assay
The activity of superoxide dismutase was determined using a diagnostic kit produced by ZellBio Company, according to Arthur and Boyne (32), and the results have been stated as units (U) of superoxide dismutase (SOD) activity per mL.
3.5.4. Serum Glutathione Peroxidase Activity Assay
The activity of glutathione peroxidase was measured using the glutathione peroxidase (GPx) kit (ZellBio Company).
In this method, the amount of sample that catalyzes 1 μmol of glutathione (GSH) to GSSG in 1 minute is expressed as a unit of GPX activity. Results have been stated as U of GPx activity per mL.
3.5.5. Assessment of Serum (GSH) Content
The evaluation of serum GSH was done using the protocol designed by Tipple and Rogers (33). Briefly, in this protocol, the oxidation of GSH by 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) leads to the formation of GSSG and 5-thio-2-nitrobenzoic acid (TNB). Then, GSSG is reduced to GSH by glutathione reductase enzyme. In this step, the rate of TNB formation is proportional to the amount of GSH and GSSG present in the sample, which is determined by measuring TNB at 412 nm.
3.5.6. Serum Contents of Pro-inflammatory Cytokines of Interleukin 2 and 6
The amount of serum IL-2 and IL-6 were measured according to ELISA kits of Abcam Company.
3.6. Histopathological Examination
3.6.1. Morphometric Studies
After fixation of tissues in phosphate-buffered formalin, the tissues were embedded in paraffin, sectioned at 5 µm, and then stained with the hematoxylin and eosin (H&E) staining method for light microscopic observations. For morphometric studies, 20 fields of each slide were randomly observed for the following criteria: Villus height and crypts depth measurements were done from the baseline of the villus using an objective lens of magnification × 10 and total magnification of × 100. Motic Images Plus 3.0 Digital Camera Software was used for morphometric studies.
3.6.2. Quantitative Histopathological Studies
The mucosal damage of the duodenum and jejunum was graded using the intestinal injury score as described by Kesik et al. (34). In this regard, the following criteria were investigated: (1) loss of crypt architecture; (2) fusion of villus; (3) widening of villus; (4) crypt loss; (5) dilatation of blood vessels; (6) cyst formation in lamina propria; (7) infiltration of neutrophils in lamina propria; and (8) cuboidal cells of enterocytes. For each parameter, to compare the severity of the damage, a 3-point scoring was used: (1) mild damage; (2) moderate damage; and (3) severe damage. Healthy tissues scored as 0 points (34).
3.7. Statistical Analysis
The normality of the data was assessed using the Shapiro-Wilk test, and then the significance of differences between the groups was determined using a one-way analysis of variance (ANOVA), followed by the Tukey post hoc test for multiple comparisons. Data are expressed as mean ± SD. Statistical analysis was done by graph Prism 5.0 (San Diego, CA, USA). P values less than 0.05 were considered statistically significant.
4. Results
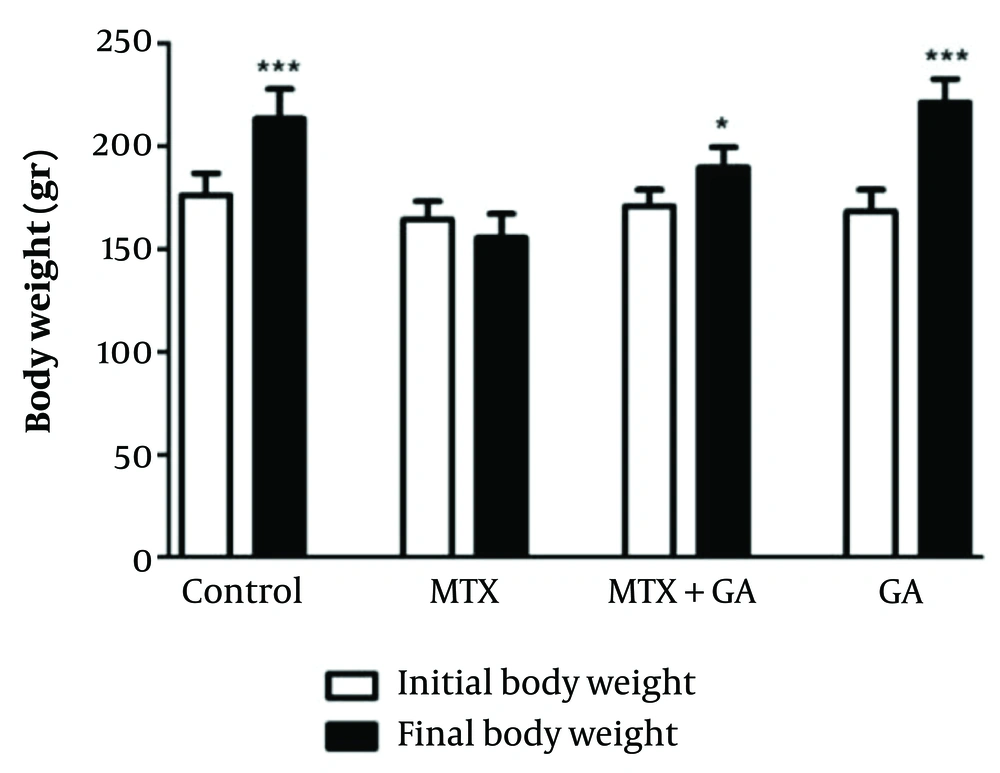
4.1. Effect of MTX and Gallic Acid on Rat Body Weights
The results obtained from rat body weights of different groups at the beginning and end of the study are illustrated in Figure 1. The control rats demonstrated a significant increase in final body weight (P < 0.001) compared to initial weights. Gallic acid supplementation also showed a significantly higher level of final body weight than initial body weight (P < 0.001). Additionally, the data of the MTX-GA rats showed a trend toward an increase in final body weight compared to initial body weight (P < 0.05).
4.2. Effect of MTX and Gallic Acid on the Serum Content of MDA
The results of the analyzed data showed a significantly higher amount of serum MDA in the MTX group than in the control group (P < 0.001; Table 1). Administration of GA before MTX could decrease this amount compared to the MTX group (P < 0.05).
| Groups | Oxidative Stress Parameters | |
|---|---|---|
| MDA (nmol/mL) | NO (nmol/mL) | |
| Control | 23.52 ± 4.21 | 20.24 ± 4.05 |
| GA | 24.47 ± 3.54 | 18.76 ± 4.12 |
| MTX | 42.75 ± 7.09 * | 65.27 ± 9.25 * |
| MTX + GA | 33.31 ± 5.27 # | 50.31 ± 7.53 ## |
Abbreviations: GA, gallic acid; MTX, methotrexate; MDA, malondialdehyde; NO, nitric oxide.
a Oxidative stress in rats was induced by a single dose of MTX (20 mg/kg bw, IP) and gallic acid (30 mg/kg bw, gavage) that was administered 6 days prior to MTX treatment and continued till the end of the experiment (4 days). Values are means ± SD (n = 7).
b Significant change with respect to the control group (* P < 0.001).
c Significant change with respect to MTX group (# P < 0.05; ## P < 0.01).
d The results show that gallic acid attenuated MTX-induced oxidative stress.
4.3. Effect of MTX and Gallic Acid on Serum Nitric Oxide Concentration
The obtained results indicated that nitric oxide (NO) concentration was significantly higher in the serum of the MTX group than in that of the control group (P < 0.001; Table 1). Again, it was seen that gallic acid significantly prevented the elevation of NO concentration when administered with MTX (P < 0.01).
4.4. Effect of MTX and Gallic Acid on the Level of SOD in the Serum of Different Experimental Groups
As seen in Table 2, the serum content of the SOD enzyme was significantly lower in the MTX group than in the control group (P < 0.001). Again, it is seen in this table that gallic acid treatment could increase this value significantly compared to the MTX group (P < 0.001).
4.5. Effect of MTX and Gallic Acid on Serum GPx Content
As shown in Table 2, administration of MTX significantly decreased the level of serum GPx content compared to the control group; thus, a P value < 0.001 was obtained. Additionally, the analyzed data showed that treatment of gallic acid could significantly increase this amount compared to the MTX group (P < 0.01).
| Groups | Antioxidant Parameters | ||
|---|---|---|---|
| SOD Activity (U/mL) | GPx Activity (U/mL) | GSH (nmol/mL) | |
| Control | 56.54 ± 7.35 | 90.34 ± 12.75 | 154.52 ± 15.21 |
| GA | 59.36 ± 7.12 | 154.26 ± 10.62 | 160.65 ± 17.06 |
| MTX | 15.27 ± 3.17 * | 50.07 ± 6.57 * | 75.95 ± 8.67 * |
| MTX + GA | 41.81 ± 5.21 # | 73.71 ± 9.67 ## | 98.02 ± 10.81 ### |
Abbreviations: GA, gallic acid; MTX, methotrexate; SOD, superoxide dismutase; GPx, glutathione peroxidase; GSH, glutathione.
a Rats were injected with MTX and gavaged with gallic acid as described in Table 1. Values are means ± SD (n = 7).
b Significant change with respect to the control group (* P < 0.001).
c Significant change with respect to MTX group (# P < 0.001, ## P < 0.01, ### P < 0.05).
d The results show that gallic acid ameliorated the MTX-induced decrease in SOD, GPx, and GSH.
4.6. Effect of MTX and Gallic Acid on Serum GSH Content of Different Rat Groups
The results obtained from the biochemical analysis indicated that the amount of GSH was 75.95 ± 8.67 in the MTX group; it was significantly lower compared to the control group (P < 0.001). The value of this parameter was 98.02 ± 10.81 in the MTX + GA group; it was statistically significant compared to the MTX group (P < 0.05; Table 2).
4.7. Effect of MTX and Gallic Acid on the Serum Level of IL-6
As shown in Table 3, the amount of serum IL-6 was 311 ± 31.20 pg/mL in the control group, while this value significantly increased to 530.15 ± 75.11 in the MTX group (P < 0.001). However, treatment with gallic acid significantly decreased (P < 0.001) the elevation of IL-6 to 326.12 ± 5.24.
| Groups | Inflammatory Parameters | |
|---|---|---|
| IL-6 (pg/mL) | IL-2 (pg/mL) | |
| Control | 311 ± 31.20 | 298.87 ± 2.20 |
| GA | 177.14 ± 2.01 | 216.42 ± 42.34 |
| MTX | 530.15 ± 75.11 * | 402.53 ± 3.12 * |
| MTX + GA | 326.12 ± 5.24 # | 245.12 ± 5.71 ## |
Abbreviations: GA, gallic acid; MTX, methotrexate; IL-6, interleukin 6; IL-2, interleukin 2.
a Rats were injected with MTX and gavaged with gallic acid as described in Table 1. Values are means ± SD (n = 7).
b Significant change with respect to the control group (* P < 0.001).
c Significant change with respect to MTX group (# P < 0.001, ## P < 0.05).
d The results show that treatment with gallic acid ameliorates the increase in inflammatory parameters caused by MTX.
4.8. Effect of MTX and Gallic Acid on the Serum Level of IL-2
The results of the analyzed data showed in the control group, the amount of serum IL-2 was 298.87 ± 2.20, and it was statistically significant compared to the MTX group (P < 0.001; Table 3).
4.9. Histopathological Findings
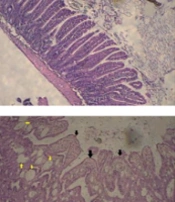
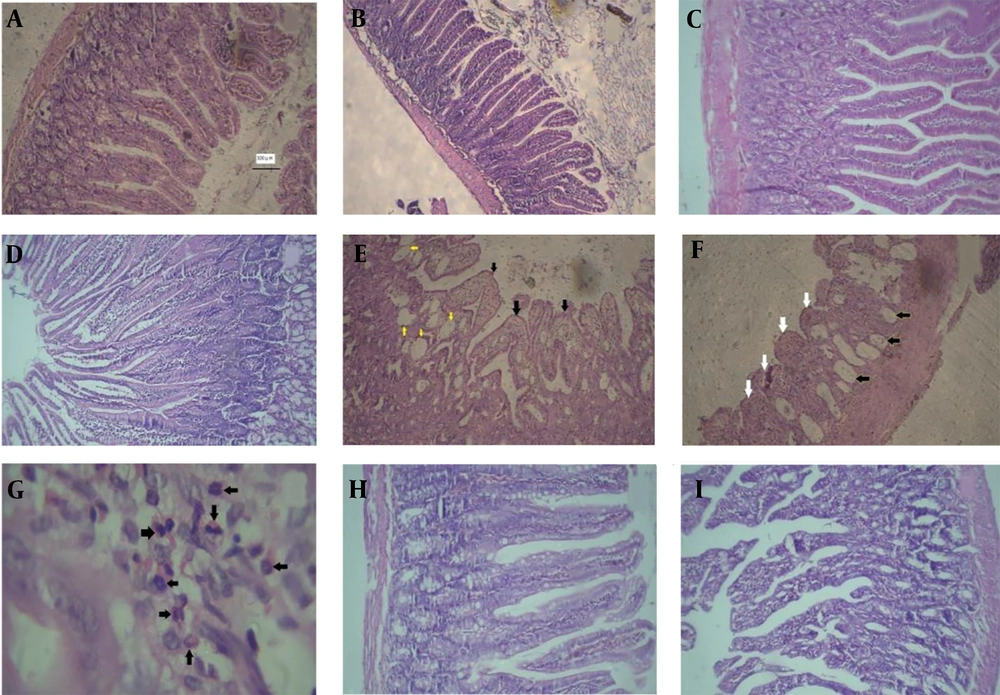
The light microscopic examination of the duodenum and jejunum slides of the control group revealed the normal architecture of the mucous layer in the form of normal epithelial enterocytes, villus, crypts, and delicate lamina propria (Figure 2A and B). A similar architecture was seen in the slides of the GA groups (Figure 2C and D). However, the observation of the slides of the MTX group showed severe damage to the mucous layer, such as shortening and fusion of villi, reduction of the height of epithelial cells, presence of round cells between enterocytes, decreased number of crypts, infiltration of neutrophils in lamina propria, cystic formation, and decreased height of villus and depth of crypts (Figure 2E, F and G). Finally, the examination of the slides of the MTX-GA group displayed almost a normal appearance similar to that of the control group (Figure 2H and I).
A and B, sections of the duodenum and jejunum of the control group notice the normal architecture of the tissues (hematoxylin and eosin (H&E) × 100); C and D, photomicrographs of the jejunum and duodenum of gallic acid (GA) group that show the normal appearance of villi, tubular crypts arranged deep in the lamina propria; E, F and G, photomicrograph of small intestine of methotrexate (MTX) group; E, cross sections of the duodenum, notice to fusion and widening of villi (black arrowheads), cysts (yellow arrowheads) (H&E × 100); F, cross-section of jejunum, intestinal cysts(black arrowheads), blunting and fusion of villi (white arrowheads), notice flattened and round cells of enterocytes (H&E × 100); G, photomicrograph of lamina propria of the duodenum that contains numerous neutrophils (black arrowheads) (H&E ×400); H and I, photomicrograph of duodenum (H) and jejunum (I) of GA-MTX group. The architecture of villi, surface epithelium, and intestinal crypts is almost normal. Note the height of villi, depth of crypts, and absence of cysts in the lamina propria (H&E × 100).
The obtained data are summarized in Table 4. Also, Table 5 shows the results of morphometric parameters.
| Fusion of Villus | Widening of Villus | Vascular Dilatation | Cyst Formation | Infiltration of Neutrophils | Crypt Loss | Epithelial Round Cells | |
|---|---|---|---|---|---|---|---|
| Duodenum | |||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GA | 0 * | 0 * | 0.20 ± 0.45 | 0 | 0.20 ± 0.45 ** | 0 | 0 * |
| MTX | 2.83 ± 0.41 # | 2.83 ± 0.41 # | 1.67 ± 1.51 | 1.67 ± 1.37 | 2.33 ± 0.82 # | 1.00 ± 0.89 | 2.83 ± 0.82 # |
| MTX-GA | 0.17 ± 0.41 * | 0.17 ± 0.41 * | 0.67 ± 0.52 | 0.17 ± 0.41 ** | 0.67 ± 0.82 | 0.50 ± 0.84 | 0.17 ± 0.41 * |
| Jejunum | |||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GA | 0 * | 0 * | 0 * | 0 * | 0.60 ± 0.89 | 0 | 0 ** |
| MTX | 2.83 ± 0.41 # | 2.67 ± 0.52 # | 2.67 ± 0.52 # | 1.83 ± 1.17 # | 1.5 ± 0.84 ## | 1.67 ± 1.21 ### | 1.67 ± 1.21 ## |
| MTX-GA | 0.83 ± 0.75 * ### | 0.83 ± 0.75 * ### | 0.83 ± 0.75 * ### | 0 * | 0.17 ± 0.41 *** | 0.33 ± 0.82 *** | 0.17 ± 0.41 ** |
Abbreviations: GA, gallic acid; MTX, methotrexate.
a Significant difference in comparison with the MTX group (* P < 0.001, ** P < 0.01, *** P < 0.05).
b Significant difference in comparison with the control group (# P < 0.001, ## P < 0.01, ### P < 0.05).
| Villus Height | Crypt Depth | |
|---|---|---|
| Duodenum | ||
| Control | 184.38 ± 23.73 | 118.74 ± 12.45 |
| GA | 178.70 ± 19.71 * | 105.43 ± 10.30 * |
| MTX | 56.50 ± 10.70 # | 48.31 ± 16.76 # |
| MTX-GA | 122.15 ± 20.14 * # | 133.95 ± 14.64 ** |
| Jejunum | ||
| Control | 207.75 ± 52.45 | 103.97 ± 9.87 |
| GA | 191.10 ± 26.22 * | 93.62 ± 6.37 * |
| MTX | 51.22 ± 20.51 # | 41.78 ± 12.51 # |
| MTX-GA | 123.16 ± 28.92 *** ## | 135.54 ± 10.61 # |
Abbreviations: GA, gallic acid; MTX, methotrexate.
a Significant difference in comparison with the MTX group (* P < 0.001, ** P < 0.05, *** P < 0.01).
b Significant difference in comparison with the control group (# P < 0.001, ## P < 0.01).
5. Discussion
Nowadays, MTX is used for the therapy of a wide range of neoplastic disorders (35), autoimmune diseases (36), liver cholestatic disorders (37), induction of abortion, etc. (18).
The objective of the current study was to examine the protective effect of GA on MTX-induced intestinal mucositis in an animal model.
The clinical manifestations of intestinal mucositis include diarrhea, mucosal ulcerations, and weight loss (38). In the current study, all of the rats who were injected with MTX experienced diarrhea and weight loss. Of course, we did not find a statistically significant relationship between the weight loss of the MTX group compared with control ones.
There is evidence that in the progression of mucositis, a complex series of biological events occurs, starting with DNA damage and the generation of reactive oxygen species (ROS) (39). The production of ROS triggers a cascade of reciprocal biological events through the activation of various transcription factors, such as nuclear factor-Kb (NF-κB) (40), which leads to increased transcription of genes associated with the progression of mucositis. These genes consist of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (41). There is concern that cytokines, especially TNF-α and IL-6, play a central role in intestinal inflammation (42). Based on in vitro studies, it was found that a low dose of IL-2 has been considered a key growth factor and essential to maintain autoimmunity (43). Additionally, due to the results of a recent clinical trial study conducted by Zhang et al., it was found that the combination of IL-2 and MTX in patients with rheumatoid arthritis led to positive improvements in immunological and clinical responses and administration of low doses of IL-2 has novel and practical therapeutic efficacy in these patients (44). So, we evaluate the amount of IL-2 and IL-6 in the serum of different study groups. The obtained results demonstrated the significant elevation of these pro-inflammatory cytokines in the serum of MTX- the animal group compared with normal ones, which is in agreement with Olsen et al. (18).
During intestinal mucositis, there is progress of inflammation, and resident monocytes contribute to the recruitment of neutrophils to the inflamed site (45). The histopathological findings of this study showed different aspects of tissue inflammation, such as dilatation of blood vessels and accumulation of neutrophils in the lamina propria of duodenum and jujena of rats exposed to MTX. Arslan et al. noticed that the aggregation of neutrophils plays a key role in the potentiating action of MTX-induced ulceration (46). Also, Kolli et al. reported that MDA and myeloperoxidase (MPO) are the marker of neutrophil activation and infiltration that increase in MTX-induced small intestine mucositis (47).
Also, the findings of the current study demonstrated that in a rat model of mucositis, there was a loss of villus architecture, fusion of villi, and loss of intervillous spaces. Similar findings were reported by some researchers (16, 19, 48, 49). Ali et al. concluded that tissue damage may be due to the folic acid analog, MTX, inhibiting DNA synthesis by binding to the DHFR enzyme, which can inhibit the proliferation of villi enterocytes in the small intestine (50).
In addition to these findings, our study showed decreased height of villi, reduced number and depth of crypts, and development of cysts. Some authors linked this outcome to DNA contents and intestinal mucosal protein (50, 51), which prevents the propagation of intestinal crypts duo to their fast division and, finally, the development of cysts (27).
In the current study, the presence of flat or cubical epithelial cells with round nuclei on the surface of some villi was observed, as previously reported by (50), and could be related to the extensive apoptosis of stem cells and anti-mitotic effect of MTX in villi (50).
Another finding of the present study was cystic formation in crypts with flat epithelial cells. This result was consistent with the (52), who explained this by inhibiting the proliferation of epithelial cells and apoptosis of stem cells in the crypts. Also, the dissimilarity in injury has been attributed to the different regional expressions of pro- and anti-apoptotic factors, such as Blc-2, which amplifies apoptosis in the crypts of the small intestine (53).
There is evidence that patients receiving antineoplastic drugs develop oxidative stress (54). Also, the administration of anti-cancer drugs can lead to a decrease in the activity of antioxidant enzymes as well as a decrease in non-enzymatic antioxidants (55). Our study confirmed that MTX administration induced oxidative stress. In this regard, the present study showed that MTX can significantly increase the serum levels of oxidative stress products such as MDA and NO. In the serum of MTX group rats, we observed a decrease in the level of endogenous antioxidants (such as GPx, GSH, and SOD), which is similar to previous studies (16).
Gallic acid is a colorless or slightly yellow crystalline compound that is widely used in the food and pharmaceutical industries (56). The most important medicinal properties of GA are attributed to its antioxidant and anti-inflammatory potential (57, 58). Our study showed that treatment with GA can improve antioxidant parameters and reduce the level of oxidative stress. These findings are consistent with previous studies (50, 59, 60). Khodayar et al. confirmed that GA can effectively reduce oxidative stress and inflammatory status in mice that have ulcerative colitis (26).
There is further evidence that GA can inhibit the secretion of pro-inflammatory mediators, nitrite, NO, and IL-6 (61). In agreement with these studies, our study showed that the administration of GA can reduce serum NO levels in MTX-induced intestinal mucositis. Sen et al., in a study on gastric ulcers induced by aspirin and pyloric ligation in rats, investigated the antiulcer effect of GA. They indicated that the high levels of MDA in rats were greatly reduced by receiving GA (62). In accordance with these results, the current study showed that treatment with GA can reduce the level of oxidative stress parameters.
According to Ghaznavi et al. (63), Kilic et al. (64), and Yang et al. (65), the current findings showed that administration of GA in rats led to an increase in enzymatic and non-enzymatic antioxidant levels, including SOD, GSH, and GPx. Also, Eslamifar et al. reported that the use of GA significantly improves kidney function with an ameliorating effect on biochemical and histopathological indicators (25).
Furthermore, the present study revealed that treatment with GA can reduce the increase in the serum content of pro-inflammatory cytokines, including IL-2 and IL-6, compared to the MTX group. Similarly, Karimi-Khouzani et al. reported the protective effect of GA on oxidative stress and inflammatory status of fluoxetine-induced hepatotoxicity (66).
Miyazono et al. (27), Xian et al. (67), and Southcott et al. (68) stated that more severe damage was caused by MTX in the jejunum than in the duodenum (27, 67, 68). The findings of our study showed that histopathological parameters are involved in both the duodenum and jejunum of rats.
In summary, an overproduction of ROS and unregulated inflammatory responses can disrupt the balance within the gastrointestinal system. This disruption can alter the typical microorganism composition and trigger a series of immediate reactions that harm cells and tissues, ultimately leading to damage to the mucous membrane and the development of mucositis (69). Regarding contextual factors, researchers should pay more attention to their inhibition with the aim of treating intestinal mucositis (16). A well-suited approach would probably focus on addressing intestinal mucositis at different stages: Utilizing antibiotics before treatment, employing antioxidants and anti-inflammatory agents during the acute phase, and promoting cell growth during the recovery phase. The researchers suggest that the most promising avenue for success lies in pursuing combination therapies that address various aspects of the intricate pathological processes associated with intestinal mucositis (70).
Experimental data indicated that compounds reach in polyphenol effectively alleviated chemotherapy agents-induced injury to intestinal epithelial cells, restrained the expression of inflammatory factors, increased cell vitality, and exerted an anti-apoptotic role on intestinal epithelial cells (71). Recent studies have indicated that the antioxidant function of GA can regulate a wide range of inflammatory cytokines, oxidative stress parameters, and enzymatic and non-enzymatic antioxidants and has the potential to be developed as a pharmaceutical agent with good therapeutic and industrial applications (72).
5.1. Conclusions
Concisely, the current study aimed to assess the beneficial effects of using GA to reduce intestinal mucositis induced by MTX in an animal model. Current study proved that GA can ameliorate various aspects of biochemical, pro-inflammatory, and histopathological damage. Finally, it seems there is a need to conduct clinical trial studies to investigate the effects of consuming a diet rich in GA on patients suffering from intestinal mucositis.