1. Background
Dementia currently affects 57.4 million people worldwide, with estimates reaching 152.8 million by 2050 (1). Alzheimer's disease (AD), prevalent in the elderly, is characterized by cognitive dysfunction, including impairments in learning and memory (2, 3). Alzheimer's disease pathophysiology involves amyloid-β (Aβ) accumulation, cholinergic dysfunction, Tau hyperphosphorylation, oxidative stress, and neuroinflammation (4-6). Key factors include microglial activation and elevated levels of proinflammatory cytokines (IL-1β, TNF-α, IL-6, IFN-γ), which disrupt cholinergic function and lead to neuronal loss (7-9). Increased levels of glial fibrillary acidic protein (GFAP), resulting from astrocyte activation, are indicative of neuronal damage (10, 11), while reduced brain-derived neurotrophic factor (BDNF) levels are associated with cognitive decline (12, 13).
Scopolamine (Sco) induces amnesia in rodents by blocking muscarinic receptors, resulting in cognitive deficits, increased acetylcholinesterase (AChE) activity, decreased acetylcholine, neuroinflammation, and elevated GFAP levels (9, 14-21). It also contributes to neurodegeneration through amyloid peptide accumulation and Tau hyperphosphorylation (9, 14, 22-24).
Current AD treatments, such as AChE inhibitors like galantamine and donepezil, can cause side effects (25). Therefore, research is focusing on safer, multitarget plant-based therapies (26, 27). Guiera senegalensis (GS), native to West and Central Africa, has shown promise in treating neurological conditions due to its antimicrobial, anticancer, antioxidant, and anti-inflammatory properties (28-36). Its neuroprotective effects have been demonstrated in zebrafish models, and further research in rodent models is ongoing to evaluate its efficacy in AD (37, 38).
2. Objectives
This research aimed to explore the anticholinesterase, antioxidant, and anti-inflammatory activities of the hydroethanolic extract of Guiera senegalensis J. F. Gmel. leaves in rats with Sco-induced dementia. The effectiveness of the treatment was assessed through behavioral tests, including the Y-maze, novel object recognition (NOR), and Morris Water Maze (MWM), which evaluate learning and memory impairments. Additionally, factors such as cholinergic dysfunction, oxidative stress, neuroinflammation, and neurodegeneration were examined using biochemical and histopathological analyses in the disease model.
3. Methods
3.1. Chemicals and Antibodies
Thiobarbituric acid (TBA), Sco, hydrogen peroxide (H₂O₂), adrenaline, and 5,5-dithiobis(2-nitro-benzoic acid) (DTNB) were obtained from Sigma-Aldrich, USA. Donepezil (Aricept®) was acquired from Pfizer, USA. ELISA kits for acetylcholine (ACh) and AChE were from BioVision, India, and Elabscience, China, respectively. ELISA kits for BDNF, amyloid-β peptide (Aβ1-42), and inflammatory markers (TNF-α, IL-1β, IL-10, IL-6, IFN-γ) were from Cusabio, China. ELISA kits for Tau phospho protein and GFAP were from Biomatik, Canada.
3.2. Plant Collection and Processing
Fresh GS leaves were collected in June 2021 from Touloum, Far North Cameroon, and deposited at the Herbier National du Cameroun as specimen No. 49837/HNC, corresponding to Sabatié B. No. 699 (G. senegalensis J. F. Gmel) (29, 39). The leaves were shade-dried for one week, ground into powder, and macerated for 72 hours in 80% ethanol and 20% distilled water. After filtering through Whatman paper, the extract was evaporated using a rotavapor at 80°C to remove ethanol, followed by drying in an oven at 50°C for 48 hours. The hydroethanolic extract yielded 7.67% (v/v) (29, 39).
3.3. In Vitro Antioxidant Activity of Hydroethanolic G. Senegalensis J. F. Gmel Leaves Extract
The in vitro antioxidant activity of GS leaves extract was evaluated by determining its ferric reducing potential and radical scavenging activity using the Ferric Reducing Antioxidant Power (FRAP) and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assays, respectively (40, 41).
3.4. Animal Models
Adult male Wistar rats (2 - 3 months old) were obtained from the Biophysics and Biochemistry Laboratory at Cameroon University. They were housed in six polyacrylic cages, with 5 rats per cage, and acclimated for 7 days in a controlled environment (25 ± 2°C, 12-hour light/dark cycles). The rats were provided with standard food and water, with a 12-hour fast before treatment and 7 hours after treatment, while water was continuously available.
3.5. Experimental Design
The six cages (n = 5) represented six groups of rats (Figure 1) Two of these groups were randomly selected as controls, one as a model, and the other three as experimental groups. The treatment protocol for each group is described below.
- Group I (control): Rats received distilled water (10 mL/kg, p.o.) via oral gavage and 0.9% NaCl (10 mL/kg, i.p.).
- Group II (model): Rats received distilled water (10 mL/kg, p.o.) via oral gavage and Sco (1 mg/kg, i.p.).
- Group III (positive control): Rats received donepezil (2 mg/kg, p.o.) via oral gavage and Sco (1 mg/kg, i.p.).
- Groups IV-VI (experimental): Sco (1 mg/kg, i.p.)-induced rats were treated with 100, 200, and 400 mg/kg of GS leaves extract, respectively, administered via oral gavage.
For administration, Sco was dissolved in saline (0.9% NaCl), and donepezil and the plant extract were dissolved in distilled water, with each rat receiving 0.1 mL/10 g. Distilled water, plant extract, or donepezil were administered orally once daily for 14 days, while Sco or saline was given intraperitoneally for 7 days (starting from day 8). Doses were based on the literature: Sco at 1 mg/kg (4, 14, 42); donepezil at 2 mg/kg (42). Cognitive functions were assessed 30 minutes after Sco or saline and 1 hour after the other treatments. The study adhered to the Cameroon Bioethics Committee and NIH guidelines, with approval from the Ethics Consultative Commission of Maroua University (Ref. No. 14/0261/Uma/D/FS/VD-RC).
3.6. Behavioral Studies
3.6.1. Y-maze Test
The Y-maze test assesses spatial short-term memory in rodents by measuring spontaneous alternations in a three-arm maze (43, 44). Each arm measured 33 cm × 11 cm × 12 cm and was arranged at 120° angles. Rats were placed at the end of one arm and allowed 8 minutes to explore. To minimize olfactory cues, the maze was cleaned with 70% ethanol between trials. Arm visit sequences were recorded during the final 5 minutes of the test. Locomotor activity was measured by counting the number of arm entries, and spontaneous alternations (consecutive entries into all three arms) were calculated as a percentage (20).
3.6.2. Test of Novel Object Recognition
The NOR test followed a standard protocol in a 72 cm x 72 cm x 36 cm open-field setup. It was conducted over three days, with each day dedicated to the habituation, training, and retention phases (2). On day 1 (habituation), rats were allowed to explore the empty field for 10 minutes. On day 2 (training), two identical objects were placed in the field, and the rats explored for 5 minutes. On day 3 (retention), one object was replaced with a novel object, while all other conditions remained the same. The setup and objects were cleaned with 70% ethanol between sessions. Exploratory behavior was recorded as the time spent exploring the novel (N) and familiar (F) objects. The Discrimination Index (DI) was calculated as follows (47):
3.6.3. Test of Morris Water Maze
Standard MWM protocols were used to assess hippocampal-dependent spatial learning and memory in rats (45, 46). The maze, a metallic cylinder with a 79 cm diameter, was divided into four quadrants (North, South, East, West) and filled with opaque water mixed with non-toxic dye. The experiment, spanning eight days, included four phases:
- Phase 1 (days 1 - 3): Rats underwent four trials each day, with the escape platform moved to different quadrants every 60 seconds. Rats that did not find the platform within 60 seconds were guided to it and left there for 20 seconds to learn its location.
- Phase 2 (days 4 - 6): The escape platform was relocated to a different quadrant, and the same protocol was followed.
- Phase 3 (day 7): Rats were placed randomly in any quadrant with its designated escape platform, and the time spent in each quadrant was recorded.
- Phase 4 (day 8): The escape platform was placed in one of the two previously unused quadrants, and the escape latency was recorded for each trial.
3.7. Biochemical Studies
Rats were euthanized using intraperitoneal injections of ketamine/diazepam (50 mg/kg / 10 mg/kg) (46). After decapitation, the brains were removed, and the hemispheres were separated. One hemisphere was fixed in 10% formaldehyde for hippocampal histology, while the other hemisphere was homogenized in 10% (w/v) ice-cold phosphate buffer (0.2 M; pH 7.4), centrifuged at 3000 rpm for 15 minutes, and the supernatant was stored at -20°C. The supernatant was used to measure Superoxide Dismutase (SOD) and Catalase (CAT) activities, as well as levels of ACh, AChE, Glutathione (GSH), Malondialdehyde (MDA), nitrite, Interleukin-1 beta (IL-1β), Tumor Necrosis Factor-alpha (TNF-α), BDNF, Interferon-gamma (INF-γ), Interleukin-10 (IL-10), Interleukin-6 (IL-6), Amyloid-beta 1-42 (Aβ1-42), Tau phospho protein, and GFAP.
3.7.1. Determination of Cholinergic Activity
Acetylcholine and AChE levels were quantified using commercially available ELISA kits, following the manufacturer's protocol, and the results were expressed in pg/mL.
3.7.2. Determination of Oxidative and Nitrosative Stress
Oxidative stress was evaluated by measuring SOD (47) and CAT activities, as well as GSH (48) and MDA (49) levels. The nitrite level was determined using the Griess method (50).
3.7.3. Determination of Neurological Inflammation and Damage
The expression of markers for inflammation (IL-1β, INF-γ, IL-6, IL-10, and TNF-α), neuronal damage (BDNF, GFAP), and AD (Aβ1-42, tau phospho protein) were assessed using commercially available ELISA kits, following the manufacturer's protocol, and their concentrations were expressed in pg/mL.
3.8. Histopathological Study and Statistical Analysis
Rat brain tissues were fixed in 10% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm thickness. The sections were stained with hematoxylin and eosin (51) and examined under a microscope at 400X magnification. The number of healthy CA1, CA2, CA3, and dentate gyrus cells was quantified using ImageJ software.
Statistical analysis was performed using GraphPad Prism version 8.0.1. One-way ANOVA followed by Tukey post-hoc tests was used for Y-maze (alternation percentage), MWM (time spent in the target quadrant, latency), NOR (DI), and biochemical parameters. Two-way ANOVA with Bonferroni post-hoc tests was applied for object recognition test exploration time and hippocampal healthy cell counts. Data are presented as mean ± SEM, and a P-value < 0.05 was considered statistically significant.
4. Results
4.1. Effect of Hydroethanolic Extract of Guiera Senegalensis J. F. Gmel. Leaves on Spatial Short-Term Memory in the Y-maze Test
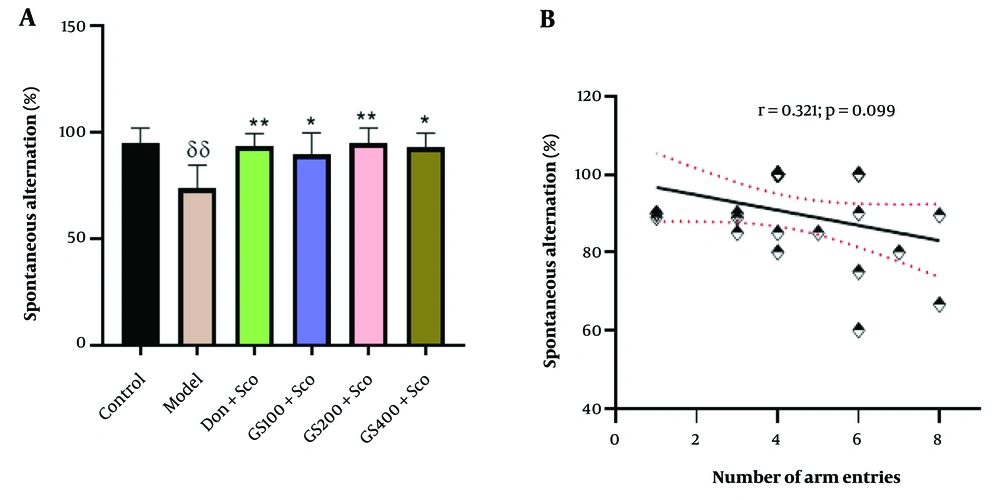
Spontaneous alternations in the Y-maze test are represented in Figure 2A. A significant difference between groups was observed [F (5, 24) = 5.01; P = 0.003]. Tukey’s post-hoc analysis revealed a noteworthy increase in spontaneous alternation in the control group compared to the Sco-treated (model) group (P < 0.01). Additionally, rats pre-treated with the hydroethanolic GS leaves extract (100, 200, and 400 mg/kg) exhibited a significant increase in spontaneous alternation compared to the Sco-treated group (P < 0.05, P < 0.01, and P < 0.05, respectively). Notably, spontaneous alternation significantly increased in the group administered donepezil (P < 0.01). Pearson's correlation (r = 0.321; P = 0.099) indicated no significant correlation between spontaneous alternation and the number of arm entries (Figure 2B).
Effect of hydroethanolic GS leaves extract on spontaneous alternation in the Y-maze test (A). Correlation analysis between spontaneous alternation and number of arm entries (Pear-son’s correlation) (B). Results are presented as mean ± SEM, (n = 5). δδ P < 0.01 significant difference from control group; * P < 0.05 and ** P < 0.01 significant difference from model group; Sco: Sco-polamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
4.2. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Long-Term Memory in the Novel Object Recognition Test
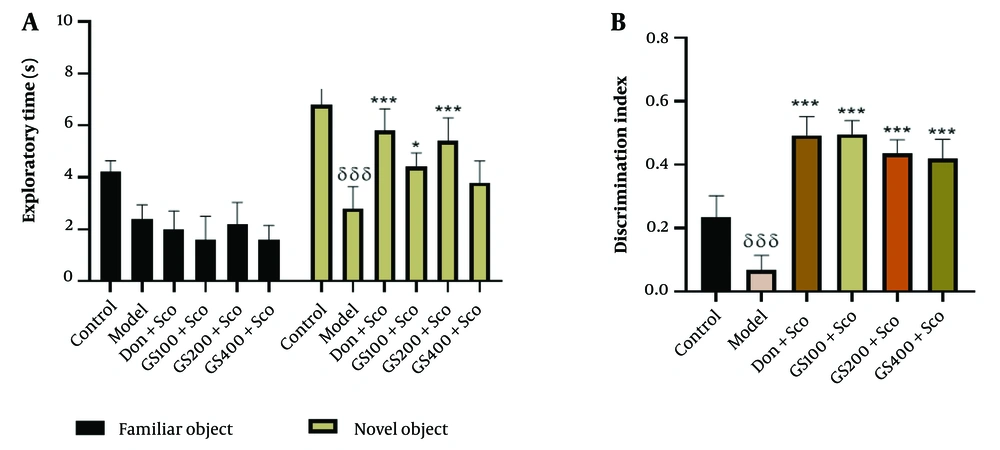
The results of the two-way analysis of variance, followed by Bonferroni post-hoc analysis, revealed a significant decrease in the time spent exploring the novel object in the Sco-treated group (model group) compared to the control group [F (5, 48) = 6.07; P < 0.001]. Conversely, the donepezil-treated group showed a significant increase (P < 0.001) in the time spent exploring the novel object (Figure 3A). The groups treated with hydroethanolic GS leaves extract at 100 and 200 mg/kg exhibited a significant increase (P < 0.05 and P < 0.001, respectively) in the time spent exploring the novel object compared to the Sco-treated group.
Effect of hydroethanolic extract of GS leaves on the exploratory time (A) and the discrimination index (B) in the novel object recognition (NOR) test. Results are represented as mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; * P < 0.05 and *** P < 0.001 significant difference from the model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senega-lensis leaves extract in mg/kg.
Figure 3B demonstrates poor object recognition in the Sco-treated group compared to the control group, as indicated by a significant (P < 0.001) decrease in the DI. This impairment was significantly reversed [F (5, 48) = 6.07; P < 0.001] by all doses of the hydroethanolic GS leaves extract, as well as in the donepezil-treated group.
4.3. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Visuospatial Learning and Memory in the Morris Water Maze Test
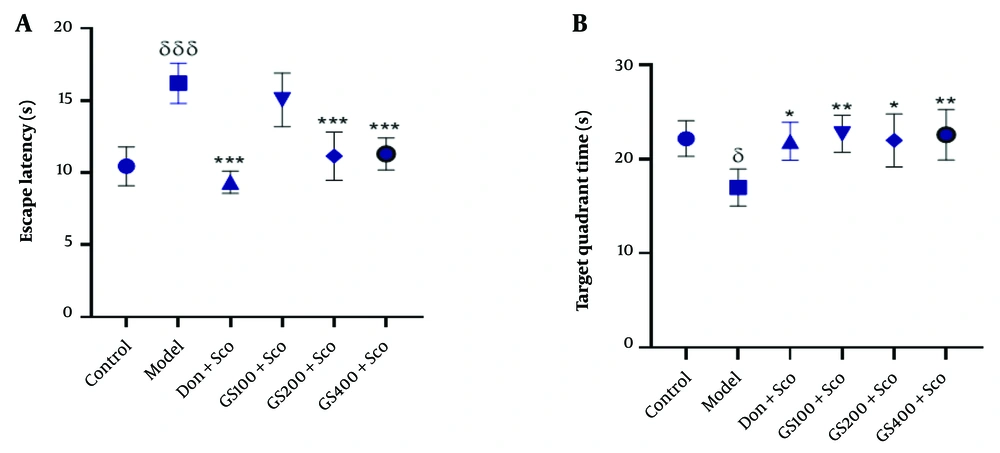
Figure 4A shows the escape latency in the MWM test. Scopolamine significantly increased escape latency in the model group compared to controls [F (5, 24) = 18.8; P < 0.001]. Pre-treatment with GS extract (100 and 200 mg/kg) and donepezil significantly reduced escape latency (P < 0.001).
Effect of hydroethanolic GS leaves extract on the escape latency (A) and the time spent in target quadrant (B) in the MWM test. Results are represented as mean ± SEM, (n = 5). δ P < 0.05 and δδδ P < 0.001 significant difference from control group; * P < 0.05, ** P < 0.01 and *** P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
Figure 4B indicates that the time spent in the target quadrant was significantly reduced in the Sco group compared to controls [F (5, 24) = 4.69; P < 0.05]. GS extract (100, 200, and 400 mg/kg) significantly increased the time spent in the target quadrant (P < 0.01, P < 0.05, and P < 0.01, respectively), as did donepezil (P < 0.01).
4.4. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Cholinergic Activity
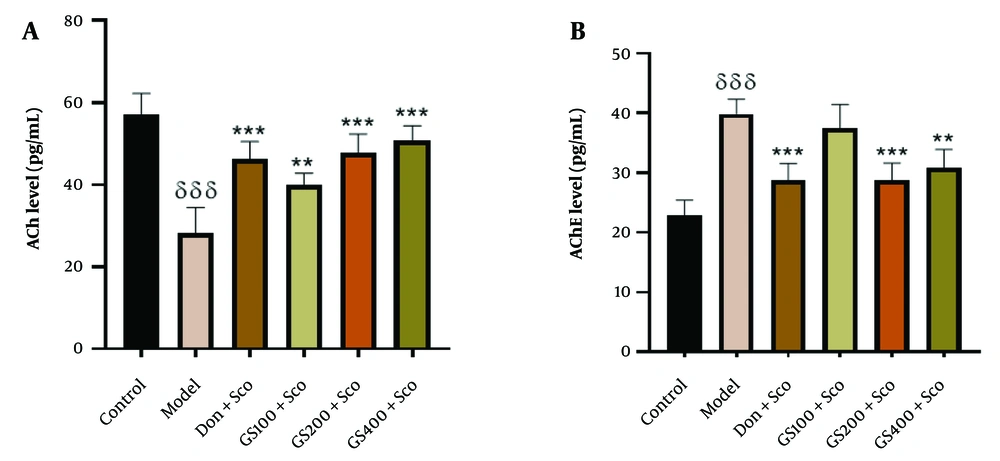
To investigate the mechanism of GS in reducing Sco-induced memory impairment, cholinergic markers were measured. Scopolamine significantly reduced brain ACh levels in the model group compared to controls [F (5, 24) = 23.6; P < 0.001]. Donepezil significantly increased ACh levels (P < 0.001). Guiera senegalensis extract pre-treatment (100, 200, 400 mg/kg) significantly increased ACh levels compared to the model group (P < 0.01, P < 0.001, and P < 0.001, respectively) (Figure 5A).
Effect of hydroethanolic GS leaves extract on hippocampus acetylcholine (A) and acetylcholinesterase (B) levels. Results are represented as mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; ** P < 0.01 and *** P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
Acetylcholinesterase levels were elevated in the model group [F (5, 24) = 21.9; P < 0.001], and donepezil also increased AChE levels (P < 0.001). GS extract (200 and 400 mg/kg) significantly reduced AChE levels (P < 0.001, P < 0.01) compared to the model group. No significant difference was observed with the 100 mg/kg dose (Figure 5B).
4.4.1. Malondialdehyde Levels
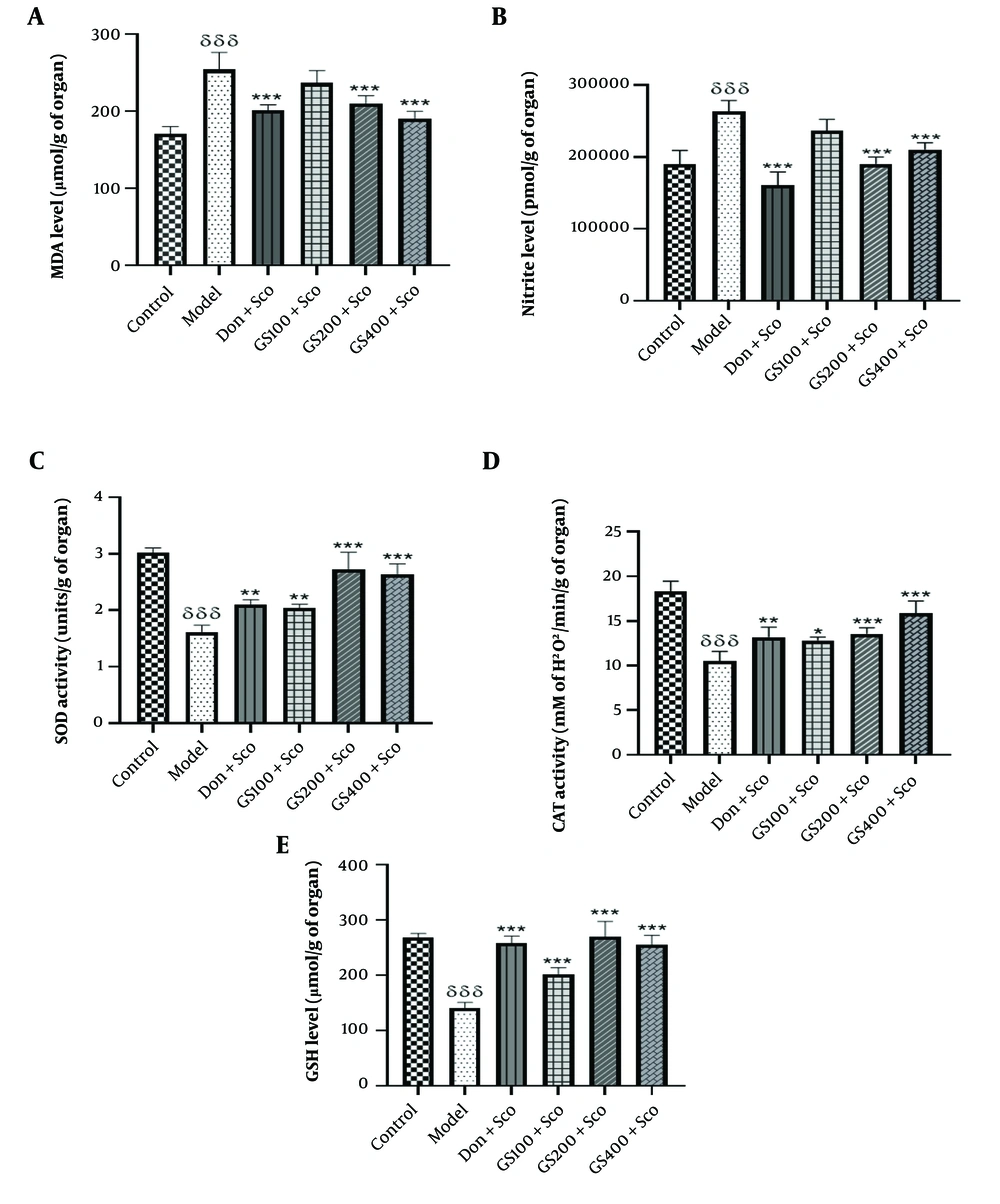
The MDA levels were notably higher [F (5, 24) = 25; P < 0.001] in rats treated with Sco (model group) compared to the control group. Conversely, the donepezil-treated group exhibited a significant reduction in MDA levels (P < 0.001). Treatment with GS extract at doses of 100 and 200 mg/kg mitigated Sco-induced lipid peroxidation, as evidenced by a significant reduction in MDA levels (P < 0.001) in these groups compared to the model group. However, no significant difference was observed between the rats receiving the extract at a dose of 100 mg/kg and the model group (Figure 6A).
Effect of hydroethanolic GS leaves extract on hippocampus malondialdehyde (MDA) (A); nitrite (B); Superoxide Dismutase (SOD) (C); Catalase (CAT) (D); and Glutathione (GSH) (E); levels. Each point represents the mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; * P < 0.05, ** P < 0.01 and *** P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
4.4.2. Nitrite Levels
As shown in Figure 6B, nitrite levels significantly increased in the model group [F (5, 24) = 28.9; P < 0.001] compared to controls. Donepezil treatment significantly reduced nitrite levels (P < 0.001). Pre-treatment with GS extract at 200 and 400 mg/kg also significantly decreased nitrite levels (P < 0.001), though the 100 mg/kg dose did not show a significant difference from the model group.
The elevated nitrite levels in the model group emphasize the role of oxidative and nitrosative stress in Sco-induced cognitive impairment. Donepezil and GS extract (200 and 400 mg/kg) effectively reduced nitrite levels, demonstrating their antioxidative properties and potential therapeutic benefits for neuroinflammation and neurodegeneration. These findings support the potential of GS extract in treating AD and related disorders.
4.4.3. Superoxide Dismutase Activity
Superoxide dismutase activity was significantly lower in the model group [F (5, 24) = 51.4; P < 0.001] compared to controls (Figure 6C). Administration of GS extract significantly increased SOD activity (P < 0.01, P < 0.001, and P < 0.001 for the 100, 200, and 400 mg/kg doses, respectively), as did donepezil (P < 0.01).
The decrease in SOD activity in the model group underscores the role of oxidative stress in Sco-induced cognitive impairment. Guiera senegalensis extract and donepezil effectively restored SOD activity, enhancing antioxidant defenses and reducing oxidative damage. These findings highlight the potential of GS extract in addressing oxidative stress-related neurodegenerative diseases, such as AD, by boosting SOD activity and protecting cognitive function.
4.4.4. Catalase Activity
Figure 6D shows a significant decrease in CAT activity [F (5, 24) = 35.7; P < 0.001] in the model group compared to controls. Pre-treatment with GS extract at 100, 200, and 400 mg/kg significantly increased CAT activity (P < 0.01, P < 0.001, and P < 0.001, respectively). Donepezil also significantly increased CAT activity (P < 0.01). The decrease in CAT activity in the model group highlights the role of oxidative stress in Sco-induced cognitive impairment. Both GS extract and donepezil effectively restored CAT activity, enhancing antioxidant defenses and reducing oxidative damage. These results suggest the potential of GS extract in treating oxidative stress-related neurodegenerative diseases, such as AD, by boosting CAT activity and protecting cognitive function.
4.4.5. Glutathione Levels
A significant decrease in GSH levels [F (5, 24) = 50.3; P < 0.001] was observed in the Sco group (model group) compared to the control group. In contrast, GSH levels in GS-treated rats (100, 200, and 400 mg/kg) were significantly higher (P < 0.001) compared to the model group, indicating the amelioration of Sco-induced oxidative stress (Figure 6E). The standard drug donepezil also significantly (P < 0.001) reversed the effect of Sco on GSH levels.
4.5. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Proinflammatory and Anti-inflammatory Cytokines Levels
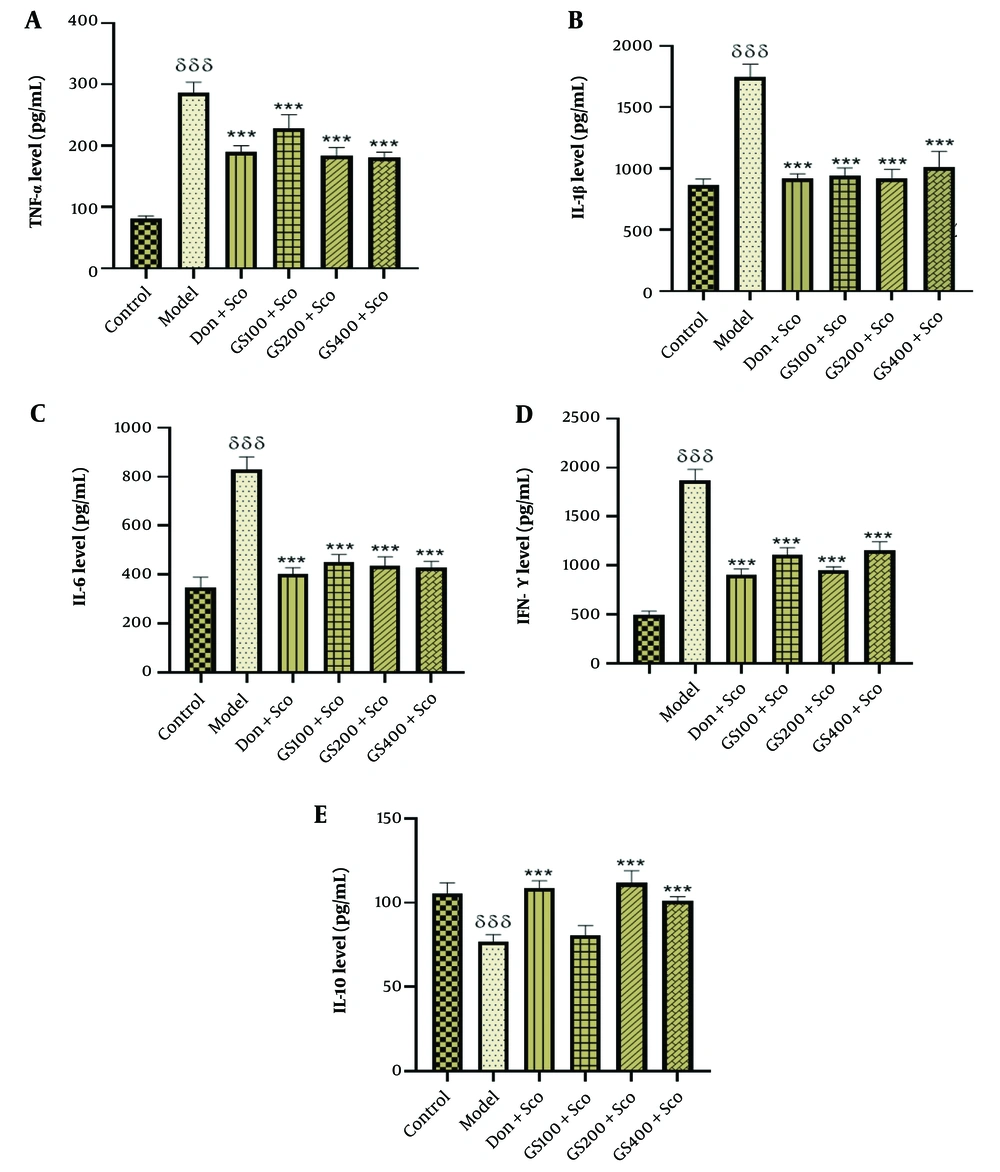
Figure 7A shows a significant increase in hippocampal TNF-α levels [F (5, 24) = 116; P < 0.001] in the Sco-treated model group compared to controls. GS extract (100, 200, and 400 mg/kg) and donepezil significantly reduced TNF-α levels (P < 0.001).
Effect of hydroethanolic GS leaves extract on hippocampus proinflammatory cytokine (TNF-α, IL-1β, IL-6, IFN-γ) and anti-inflammatory cytokine (IL-10) levels. Each point represents the mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; *** P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
Figure 7B indicates higher hippocampal IL-1β levels [F (5, 24) = 82.7; P < 0.001] in the model group. The GS extract (100, 200, and 400 mg/kg) and donepezil significantly decreased IL-1β levels (P < 0.001).
Figure 7C shows increased IL-6 levels [F (5, 24) = 25; P < 0.001] in Sco-treated rats. The GS extract (100, 200, and 400 mg/kg) and donepezil significantly reduced IL-6 levels (P < 0.001).
Figure 7D reveals a significant increase in IFN-γ [F (5, 24) = 190; P < 0.001] in the Sco group. The GS extract (100, 200, and 400 mg/kg) and donepezil significantly lowered IFN-γ levels (P < 0.001).
Figure 7E shows lower IL-10 levels [F (5, 24) = 39.1; P < 0.001] in the model group. The GS extract (200 and 400 mg/kg) and donepezil significantly increased IL-10 levels (P < 0.001). No significant difference was found between the 100 mg/kg GS dose and the model group.
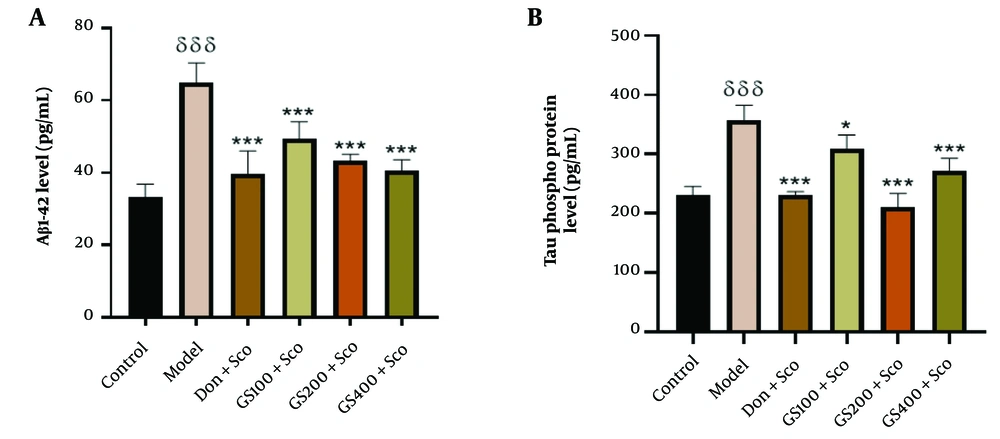
4.6. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Amyloid-beta 1-42 (Aβ1-42) and Tau Phospho Protein Levels
4.6.1. Amyloid-beta 1-42 (Aβ1-42) Level Estimation
Figure 8A shows that Aβ1-42 levels were significantly higher [F (5, 24) = 31.0; P < 0.001] in the model group compared to controls. Guiera senegalensis extract (100, 200, and 400 mg/kg) and donepezil significantly reduced Aβ1-42 levels (P < 0.001). The elevated Aβ1-42 levels in the model group underscore the role of amyloid beta peptides in Sco-induced cognitive impairment and neurodegeneration. The effectiveness of GS extract and donepezil in reducing Aβ1-42 levels suggests their potential in mitigating AD pathology, offering neuroprotective effects and aiding in the preservation of cognitive function. These results emphasize the importance of targeting amyloid beta peptides in AD treatment strategies.
Effect of hydroethanolic GS leaves extract on effect on hippocampus Aβ1-42 (A) and phospho Tau protein (B) levels. Results are represented as mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; * P < 0.05 and *** P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
4.6.2. Tau Phospho Protein Level Estimation
Figure 8B shows significantly increased phospho Tau protein levels [F (5, 24) = 38.2; P < 0.001] in the model group compared to controls. Pre-treatment with GS extract (100, 200, and 400 mg/kg) significantly reduced these levels (P < 0.05, p < 0.001, and P < 0.001, respectively), and donepezil also significantly reduced phospho Tau protein levels (P < 0.001). The elevated phospho Tau in the model group underscores the role of tau pathology in Sco-induced cognitive impairment. The GS extract and donepezil effectively reduced phospho Tau levels, suggesting their potential in mitigating tau pathology and preserving neuronal function in AD. These findings highlight the importance of targeting tau pathology for AD treatment.
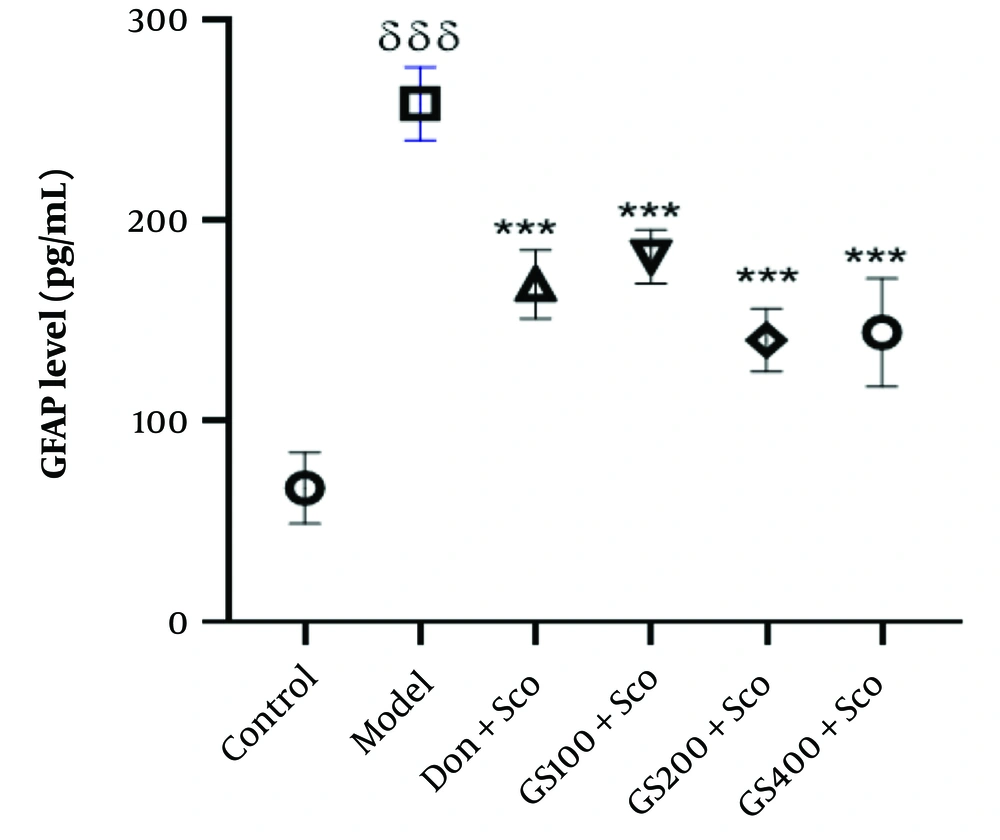
4.7. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel Leaves Extract on Glial Fibrillary Acidic Protein Level
Figure 9 shows significantly elevated GFAP levels [F (5, 24) = 56.2; P < 0.001] in the model group compared to controls. Treatment with GS extract (100, 200, and 400 mg/kg) and donepezil significantly reduced GFAP levels (P < 0.001). The increased GFAP in the model group indicates neuroinflammation and neuronal damage associated with Sco-induced dementia. Both GS extract and donepezil effectively decreased GFAP levels, suggesting their potential to attenuate neuroinflammation and protect against neuronal damage. These results highlight the value of targeting neuroinflammation as a therapeutic strategy for AD and related disorders.
Effect of hydroethanolic GS leaves extract on hippocampus GFAP level. Results are represented as mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; ***P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Concentration of Guiera senegalensis leaves extract in mg/kg.
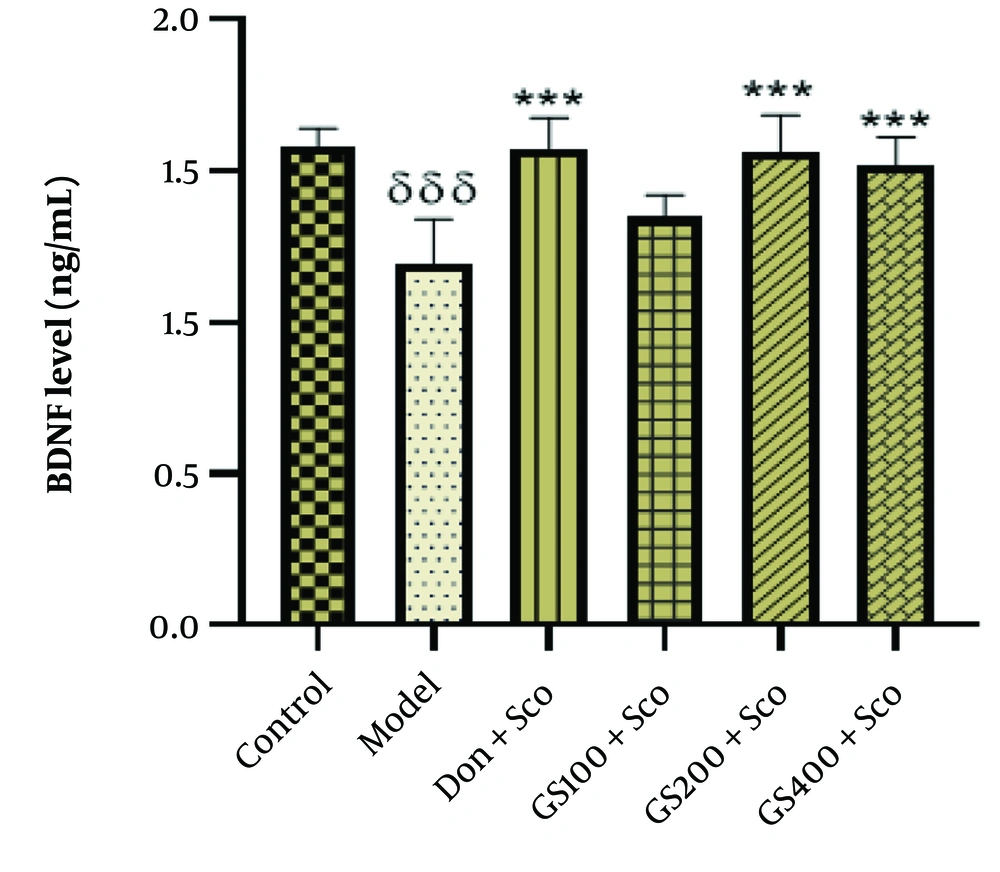
4.8. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Brain-Derived Neurotrophic Factor Level
Figure 10 shows a significant reduction in BDNF levels [F (5, 24) = 11.5; P < 0.001] in the model group compared to controls. Treatment with GS extract (200 and 400 mg/kg) and donepezil significantly increased BDNF levels (P < 0.001), though the 100 mg/kg dose of GS did not show a significant difference from the model group. The decreased BDNF in the model group highlights impaired neurotrophic support and synaptic plasticity in Sco-induced dementia. The GS extract and donepezil effectively increased BDNF levels, suggesting their potential to enhance neurotrophic signaling, promote neuronal survival, and improve cognitive function. These findings underscore the importance of boosting neurotrophic support as a therapeutic approach for AD and similar neurodegenerative conditions.
Effect of hydroethanolic GS leaves extract on on hippocampus brain-derived neurotrophic factor (BDNF) level. Results are represented as mean ± SEM, (n = 5). δδδ P < 0.001 significant difference from control group; *** P < 0.001 significant difference from model group; Sco: Scopolamine 1 mg/kg, Don: Donepezil 2 mg/kg, GS: Con-centration of Guiera senegalensis leaves extract in mg/kg.
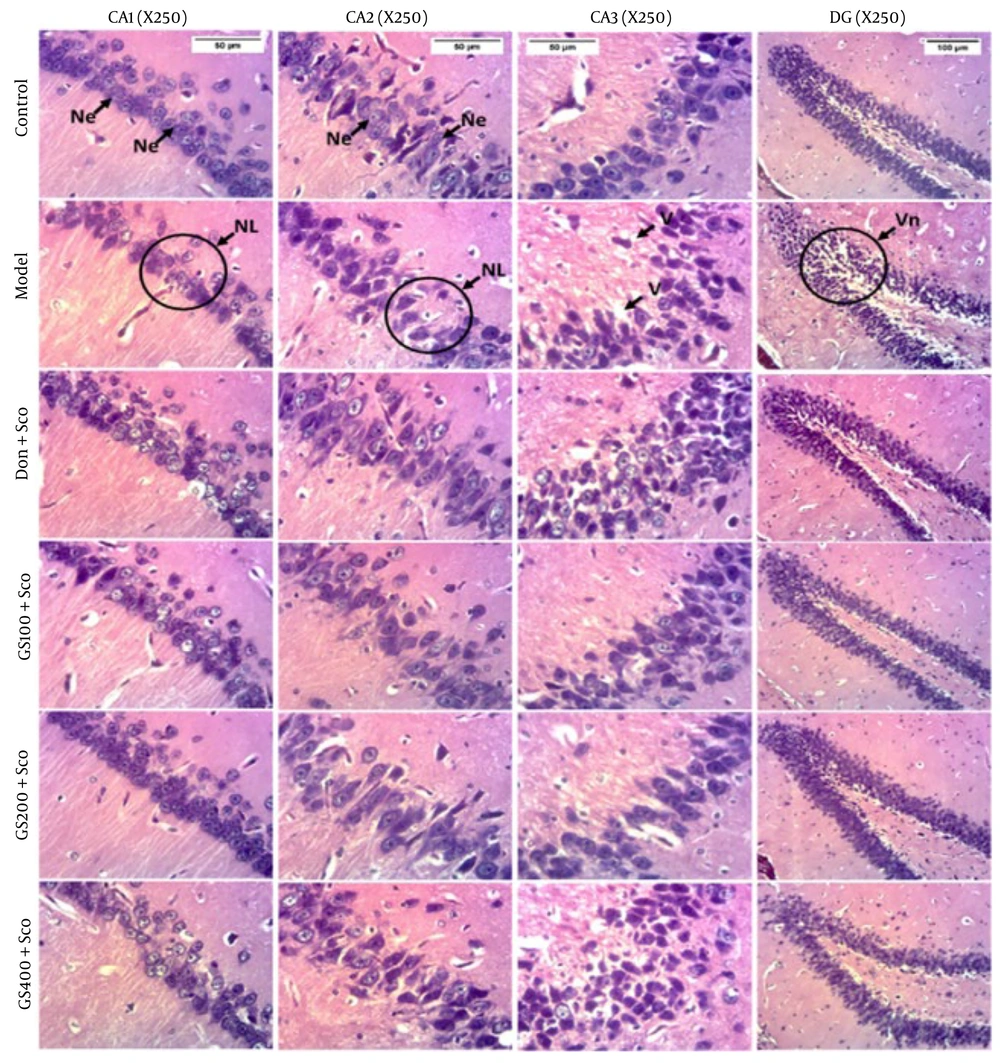
4.9. Effect of Hydroethanolic Guiera Senegalensis J. F. Gmel. Leaves Extract on Histological Sections
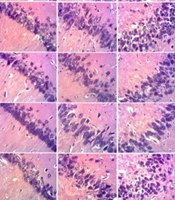
Figure 11 shows that the control group exhibited normal hippocampal microarchitecture in the CA1, CA2, CA3 regions, and the dentate gyrus (DG). Scopolamine treatment for 7 days caused a marked decrease in cell density in these areas. Pre-treatment with GS extract and donepezil reversed these changes, indicating their potential neuroprotective effects. These findings suggest that both GS extract and donepezil can mitigate neuronal damage and preserve hippocampal integrity, offering promise for therapeutic interventions in AD and similar neurodegenerative disorders.
Effect of hydroethanolic GS leaves extract on scopolamine (Sco)-induced hippocampus histological changes. Coronal sections - hematoxylin and eosin - magnification 250X. CA1 = Cornu ammonis 1, CA2 = Cornu ammonis 2, CA3 = Cornu ammonis 3, DG = Dentate gyrus. Ne = Neuron, NL = Neuronal Loss, V = Vacuole, Vn = Vacuolated neuron. Sco = Scopolamine 1 mg/kg, Don = donepezil 2 mg/kg, GS = concentration of Guiera senegalensis leaves extract in mg/kg
5. Discussion
The Sco-induced dementia model is commonly used to study cognitive impairments and therapies for AD (9, 14, 15). This study evaluated the effects of the hydroethanolic extract of GS leaves on cholinergic dysfunction, oxidative stress, neuroinflammation, neurodegeneration, and memory impairment in Wistar rats with Sco-induced dementia.
Behavioral tests (Y-maze, NOR, MWM) demonstrated that GS extract improved short-term, long-term, and spatial memory. Specifically, GS extract and donepezil reversed Sco-induced memory deficits in the Y-maze (20, 44), enhanced long-term memory in the NOR test (43, 52), and improved spatial memory in the MWM test by reducing escape latency and increasing the time spent in the target quadrant (45, 53).
Biochemical analyses revealed that GS extract reversed Sco-induced decreases in ACh levels and increases in AChE, thereby enhancing cholinergic function (3, 14, 15, 54). The GS also reduced oxidative stress by lowering MDA levels and restoring SOD, CAT, and GSH levels (5, 19, 21, 54).
Inflammatory markers (TNF-α, IL-1β, IL-6, IFN-γ) were elevated by Sco, while IL-10 and BDNF levels were reduced, indicating neuroinflammation. The GS extract reversed these changes, suggesting anti-inflammatory and neuroplasticity benefits (4, 10, 11, 55). Additionally, GS reduced Aβ1-42 and phospho Tau levels, indicating potential therapeutic benefits by inhibiting amyloid plaque formation and Tau hyperphosphorylation (56-58).
The antioxidant and anti-inflammatory effects of GS are likely attributed to its bioactive compounds, such as flavonoids and polyphenolic acids (19, 46, 59, 60). Compounds like quercetin, apigenin, and catechin have shown promise in improving memory and cognitive function (9).
Histological analysis confirmed that GS extract reversed the Sco-induced decrease in cell density in the hippocampus, aligning with the behavioral and biochemical findings. Overall, hydroethanolic GS extract effectively mitigates Sco-induced cognitive impairments, highlighting its therapeutic potential for AD and similar conditions.
5.1. Conclusions
Our results suggest that the hydroethanolic extract of GS holds promise as a potential treatment for AD. Guiera senegalensis exhibited significant anticholinesterase, antioxidant, and anti-inflammatory effects, improving cognitive function while reducing oxidative stress and neuroinflammation in a Sco-induced rat model. The reduction in anticholinergic activity coincided with decreased levels of pro-inflammatory cytokines and oxidative stress markers, indicating potential interactions between these pathways. These findings highlight GS’s multifaceted neuroprotective effects and its potential as a therapeutic agent for AD, warranting further exploration of its mechanisms and clinical applications.