1. Background
The Artemisia genus (family Asteraceae) contains more than 498 species worldwide, primarily distributed across Europe, Asia, and North America (1, 2). In Iran, this genus includes 34 species that grow wild in dry or semi-dry habitats (1, 2). The phytochemicals reported from Artemisia include flavonoids, coumarins, sterols, polyacetylenes, monoterpenes, sesquiterpenes, and sesquiterpene lactones (1-4). Several Artemisia species have been documented to possess antiviral, anti-inflammatory, anti-helminthic, antihepatotoxic, anti-malarial, antiseptic, cytotoxic, and antispasmodic activities (5-7). Therefore, identifying the secondary metabolites in these species could open new avenues for understanding the biological effects and nutritional properties of this herb.
Artemisia biennis, known as "Dermaneye dosaaleh" in Persian, is distributed across various regions of Iran (8). Previous studies in Iran have reported the presence of α-pinene (10.2%), 1,8-cineole (10.1%), Artemisia ketone (11.4%), and camphor (24.6%) as the main components of A. biennis essential oil (EO) (9). Lopes-Lutz et al. identified nearly 37 compounds in A. biennis EO from Canada, with the highest percentages attributed to (E)-β-farnesene (40%), (Z)-β-ocimene (34.7%), and (Z)-en-yn-dicycloether (11%) (10). Due to its high activity against dermatophytes, Cryptococcus neoformans, Fonsecaea pedrosoi, and Aspergillus niger, A. biennis EO could be a promising candidate for skin and hair care formulations (10). Additionally, the EO of A. biennis has demonstrated antibacterial activity against Staphylococcusaureus, Lactobacillus plantarum, and Escherichia coli (11). The EO from the aerial parts of A. biennis also showed significant analgesic effects in male rats, potentially involving the modulation of glutamatergic mechanisms through opioid systems (12).
Mohammadi et al. demonstrated that the vegetative state could quantitatively and qualitatively affect the EO of A. absinthium (13). The cytotoxic effects of dichloromethane extract fractions from A. biennis against MCF-7 and human prostate carcinoma (PC3) cancer cell lines have been previously reported, suggesting this plant as a potential source of cytotoxic phytochemicals (7). Therefore, a phytochemical study on the aerial parts of A. biennis, a plant growing in Iran, seems reasonable to investigate its ecological importance, nutritional value for animal feed, and to complete the study on the secondary metabolites of its EO.
2. Objectives
While A. biennis essential oils (EOs) have been studied for their biological properties, research specifically focusing on the effect of different growth stages on the chemical composition of the EO and its toxicity performance has not been previously conducted. Therefore, the primary aim of this study was to identify the chemical composition of EOs isolated from the aerial parts of A. biennis during the early vegetative stage, pre-flowering stage, full-flowering stage, and late vegetative stage from the mountainous areas of Iran for the first time. Additionally, this research aimed to evaluate the cytotoxicity of the obtained A. biennis EOs at different growth stages against various cancerous cell lines.
3. Methods
3.1. Materials
The cell culture media and supplements, including Dulbecco’s modified Eagle’s medium (DMEM-F12), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and penicillin-streptomycin, were obtained from Bonyakhteh (Iran). The human colon adenocarcinoma (HT-29), breast adenocarcinoma (MCF-7), and normal fibroblast cell lines were obtained from the Pasteur Institute (Tehran, Iran).
3.2. Preparation of Essential Oils
The aerial parts of A. biennis were collected from the mountainous areas of Jaghargh (36°18′43″N 59°19′21″E), Mashhad, Iran, in June (early vegetative stage), July (pre-flowering stage), August (full-flowering stage), and October (late vegetative stage) of 2021. The samples were identified and an herbarium specimen (No. 13605) was deposited in the herbarium of the School of Pharmacy at Mashhad University of Medical Sciences (Mashhad, Iran).
The aerial parts of the plant were washed with deionized water, dried at room temperature, and then finely powdered using an electric mill. The essential oil was extracted from the A. biennis powder through hydro-distillation using an all-glass Clevenger-type apparatus (14). Thirty grams of plant material were soaked in 300 mL of distilled water, placed in a glass flask, and heated for 3 hours at 100°C. Anhydrous sodium sulfate was used to remove any remaining water after extraction. The EO was stored in an airtight glass container at 4°C, and the EO yields were calculated as a percentage of the dry weight (% w/w). The extraction was performed three times, and the mixed EOs from each growth stage were then analyzed by GC/MS.
3.3. Gas Chromatography–mass Spectrometry (GC/MS) Analysis
Gas chromatography–mass spectrometry analysis was performed using an Agilent Technologies 7890B GC System/5977A MSD (USA) equipped with a fused silica capillary HP-5 column (30 m × 0.25 mm, 0.25 μm film thickness). Helium was used as the carrier gas at a flow rate of 1 mL per minute. The process was conducted under the following conditions: Injection volume of 1 µL with a split ratio of 1:20, injector temperature set at 280°C, and the oven temperature programmed to increase from 30°C to 280°C at a rate of 3°C per minute. Mass spectra were obtained in electron impact (EI+) mode at 70 eV, with the ion source temperature set at 230°C. Mass spectra were recorded over the 50 - 500 a.m.u. range (15). Retention indices were calculated relative to the retention times of n-alkanes (C8-C24). The EO components were identified by comparing their Kovats indices with those reported in the literature and by referencing internal libraries (Wiley, NIST, and Mass Finder 2.1 GC/MS libraries) (16).
3.4. Cytotoxicity Test
3.4.1. Cell Cultures
The HT-29, MCF-7, and normal fibroblast cell lines were maintained in DMEM-F12 medium supplemented with 10% v/v fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were sub-cultured two to three times per week (17, 18).
3.4.2. Cytotoxicity Evaluation Using MTT Assay
The MTT assay was used to investigate the toxicity of the EOs, following a standard method previously reported (17, 18). A cell suspension with a concentration of 5 × 103 cells/mL was plated in each well of 96-well plates, and 100 μL of DMEM-F12 culture medium containing 10% FBS was added to each well. The plates were incubated for 24 hours at 37°C, with 90% humidity and 5% CO2. Various concentrations of the EOs, prepared through serial dilution (100, 50, 25, 12.5, and 6.25 µg/mL), were then added to the wells and incubated for an additional 24 hours. A 1% dimethyl sulfoxide (DMSO) solution was used as a positive control. The medium was then replaced with 100 μL of MTT solution (0.5 mg/mL), followed by a further 4-hour incubation period. After removing the solution, DMSO was added to dissolve the formazan product. The color intensity, which is proportional to the number of living cells, was measured using an ELISA plate reader (BioTek, ELx800™, USA) at a wavelength of 570 nm. Each assay was performed in triplicate, and the mean and standard error of the mean (SEM) were calculated. The IC50 values were determined using Prism software.
3.5. Statistical Analysis
Each experiment was performed three times, and the results were presented as means ± SEM. A one-way analysis of variance (ANOVA) was used to compare the differences between means. A probability value of P < 0.05 was considered statistically significant.
4. Results and Discussion
4.1. Phytochemical Analysis
The aerial parts of the Artemisia genus have been used in Iranian traditional medicine for therapeutic purposes, primarily because most essential oil components accumulate in the aerial parts of the plant (5, 8, 12). Therefore, this study focused on investigating the EOs of A. biennis aerial parts at different growth stages. The average EO yields ranged from 0.29 ± 0.08% in October to 0.5 ± 0.021% in August (Table 1), following the sequence: Late vegetative stage (0.29 ± 0.08%) < early vegetative stage (0.36 ± 0.02%) < pre-flowering stage (0.4 ± 0.014%) < full-flowering stage (0.5 ± 0.021%). The highest EO yield was observed in August during the full-flowering stage of A. biennis, consistent with findings from Verdian-Rizi et al. (19) regarding Artemisia annua EO yields. Similar results were also reported by Mallavarapu et al. (20), Sellami et al. (21), Özgüven et al. (22), and Rohloff et al. (23) at the full emergence of flower heads in Artemisia pallens Wall. ex Besser, Origanum majorana L., Thymus vulgaris L., and peppermint, respectively. The decrease in EO yield during the vegetative phase of the plant is likely due to the partial inactivation of enzymes necessary for the biosynthesis of specific volatile compounds (21). Therefore, the timing of plant harvesting plays a crucial role in achieving maximum EO yield. Various factors such as the season of harvest, soil pH, plant parts used, drying conditions, chemotype, genotype, subspecies, extraction methods, and plant collection location can impact the final EO yield (5). Previous studies have documented EO yields of A. biennis species in the range of 0.3% (10) to 0.42% (9). The EO yields calculated in the current study exceeded those of other A. biennis ecotypes.
| No. | Name | June | July | August | October | Lopes-Lutz et al. (10) | Nematollahi et al. (9) |
|---|---|---|---|---|---|---|---|
| 1 | Hexanal | 0.1 | |||||
| 2 | Santolina triene | 4.9 | |||||
| 3 | α-Thujene | t a | |||||
| 4 | Benzaldehyde | 0.2 | |||||
| 5 | Sabinene | 0.1 | 0.8 | ||||
| 6 | α-Pinene | 0.37 | 0.31 | 0.52 | 0.29 | 0.2 | 10.2 |
| 7 | Camphene | 2.9 | |||||
| 8 | p-Cymene | 0.14 | 0.8 | ||||
| 9 | O-Cymene | 0.18 | |||||
| 10 | 1,8-Cineole | 0.85 | 0.29 | 10.1 | |||
| 11 | Linalool | 0.19 | 0.27 | 0.18 | |||
| 12 | β-Pinene | 0.2 | t a | 1 | |||
| 13 | (E)-α- necrodol | 3.42 | |||||
| 14 | Yomogi alcohol | 0.13 | 0.13 | ||||
| 15 | Limonene | 1.69 | 0.2 | ||||
| 16 | Limona ketone | 0.3 | 0.13 | 0.41 | |||
| 17 | β-Phellandrene | t a | |||||
| 18 | Caryophyllene oxide | 0.39 | 0.2 | ||||
| 19 | Cyclocopacamphenol | 0.85 | |||||
| 20 | Myrtenol | 0.9 | |||||
| 21 | Dihydrocarveol | 0.83 | |||||
| 22 | Photonerol | 6.37 | 3.94 | 6.8 | |||
| 23 | Terpinene-4-ol | 0.76 | 0.46 | 0.73 | 1.3 | 2.6 | |
| 24 | Trans-sabinyl acetate | 0.7 | |||||
| 25 | (E)-β-Farnesene | 14.13 | 6.89 | 11.49 | 16.38 | 40 | 1.8 |
| 26 | (Z)-α- Farnesene | 1.18 | |||||
| 27 | Germacrene D | 0.4 | 5.3 | ||||
| 28 | Cabreuva oxide A | 0.2 | |||||
| 29 | β-selinene | 1.8 | |||||
| 30 | α-selinene | 2.9 | |||||
| 31 | (E)-β-Ionone | 2.26 | 1.41 | 1.34 | 2.4 | ||
| 32 | α- Calacorene | 1.19 | |||||
| 33 | β- Maaliene | 1.49 | 0.99 | 0.58 | |||
| 34 | δ-cadinene | 0.92 | 1.02 | 0.48 | |||
| 35 | Alloaromadendrene | 0.81 | |||||
| 36 | (Z)-Nerolidol | 41.69 | 41.72 | 54.4 | 22.62 | ||
| 37 | (E)-Nerolidol | 0.45 | 0.94 | ||||
| 38 | β-Humulene | 0.37 | |||||
| 39 | (Z)-β-Ocimene | 34.7 | |||||
| 40 | (E)-β-Ocimene | 0.7 | |||||
| 41 | cis-Sabinene hydrate | 0.2 | 1 | ||||
| 42 | trans-Sabinene hydrate | 0.4 | 0.6 | ||||
| 43 | Artemisia alcohol | 0.1 | 1.4 | ||||
| 44 | Artemisia ketone | 11.4 | |||||
| 45 | α-campholenal | 0.6 | |||||
| 46 | Trans-pinocarveol | 3.8 | |||||
| 47 | Camphor | 24.6 | |||||
| 48 | Pinocarvone | 3.2 | |||||
| 49 | Borneol | 2.6 | |||||
| 50 | Allo-ocimene | 0.8 | |||||
| 51 | (Z)-Myroxide | 0.1 | |||||
| 52 | cis-Verbenyl acetate | 0.2 | |||||
| 53 | Farnesyl acetate | 0.71 | 0.6 | ||||
| 54 | (Z), (E)-Farnesyl acetate | 0.5 | |||||
| 55 | α-himachalene | 0.68 | |||||
| 56 | β-Costol | 0.73 | |||||
| 57 | γ-costol | 0.76 | |||||
| 58 | τ-Cadinol | 0.81 | 1.13 | 1.13 | |||
| 59 | Aromandendrene | 4.68 | 0.21 | ||||
| 60 | α-Bisabolol | 0.57 | 1.96 | 1.01 | |||
| 61 | β- bisabolene | 0.91 | |||||
| 62 | (Z), (Z)- Farnesol | 0.38 | 9.77 | ||||
| 63 | (E), (E)-Farnesol | 0.9 | |||||
| 64 | Valerenol | 0.56 | |||||
| 65 | β-Cyperone | 0.34 | 0.49 | 0.59 | |||
| 66 | Viridiflorol | 6.05 | |||||
| 67 | α-Terpineol | 2.12 | 1.28 | 0.55 | 1.51 | 0.2 | |
| 68 | α-Terpinene | 0.1 | |||||
| 69 | γ-Terpinene | 0.33 | 0.8 | 0.5 | |||
| 70 | (Z)-Caryophyllene | 0.33 | |||||
| 71 | (E)-Caryophyllene | 0.41 | 1.24 | 0.6 | 1.6 | ||
| 72 | γ-Gurjunene | 6.52 | |||||
| 73 | Hexahydrofarnesyl acetone | 0.35 | 1.11 | 0.76 | |||
| 74 | α- Santalol | 1.70 | 0.3 | ||||
| 75 | Intermedeol | 6 | |||||
| 76 | (Z)-tonghaosu | 12.33 | 18.61 | 16.38 | 17.06 | 10 | |
| 77 | (E)- tonghaosu | 1 | |||||
| 78 | Tibetin spiroether | 0.37 | 0.41 | 0.55 | 0.68 | ||
| 79 | Gerany-p-cymene | 0.4 | 0.88 | 1.01 | 0.81 | ||
| 80 | Phytol | 0.18 | 0.46 | 0.59 | |||
| 81 | Ascaridole | 1.5 | |||||
| 82 | Valencene | 0.81 | |||||
| 83 | Lyratyl acetate | 0.54 | |||||
| 84 | Monoterpene hydrocarbon | 2.59 | 0.45 | 0.52 | 0.47 | ||
| 85 | Oxygenated monoterpenes | 11.24 | 6.93 | 3.97 | 11.55 | ||
| 86 | Sesquiterpene hydrocarbon | 23.08 | 11.89 | 11.49 | 26.34 | ||
| 87 | Oxygenated sesquiterpenes | 48.71 | 56.99 | 63.44 | 41.15 | ||
| 88 | diterpene hydrocarbon | 0.4 | 0.88 | 1.01 | 0.81 | ||
| 89 | Oxygenated diterpene | 0.18 | 0.46 | 0.59 | |||
| 90 | Spiro Ethers | 12.7 | 19.02 | 16.93 | 17.74 | ||
| 91 | Unknown | 1.1 | 3.38 | 2.64 | 1.35 | ||
| 92 | Oil yield (%w/w) | 0.36 ± 0.02 | 0.4 ± 0.014 | 0.5 ± 0.021 | 0.29 ± 0.08 | 0.3 | 0.42 |
a t = trace (≤ 0.05%).
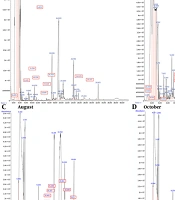
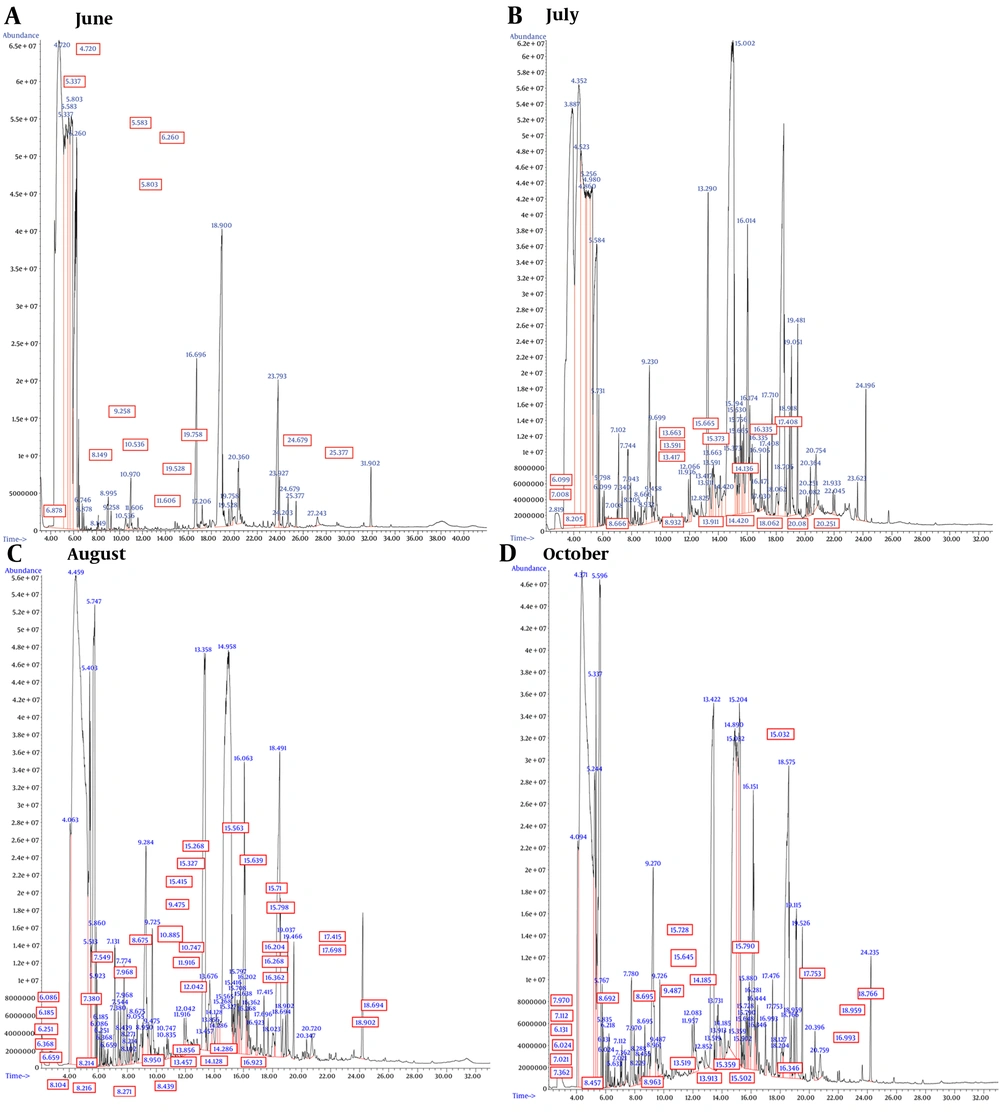
The GC/MS chromatograms of A. biennis EO are presented in Figure 1, with the corresponding results detailed in Table 1. The chemical constituents across four different collection periods (based on month) were identified, accounting for at least 96% of the total composition. The results showed that 35, 30, 11, and 32 total compounds were identified in June, July, August, and October, respectively. The chemical components of the analyzed oils were classified into monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, diterpene hydrocarbons, oxygenated diterpenes, and spiroethers. Qualitative and quantitative variations in the chemical profile of the EOs were observed across different harvesting months. Overall, the chemical composition was dominated by oxygenated sesquiterpenes, with oxygenated diterpenes being a minor fraction.
The chemical diversity of the EO constituents decreased as the growth stages progressed towards the full-flowering stage, while the yield of volatile oil extraction increased inversely. Additionally, during the first three stages of plant growth, the number of distinctive and exclusive compounds—those detected in only one growth stage—also decreased. However, in the late vegetative stage, all three trends were reversed, resulting in an increase in the chemical diversity of the EO constituents, a decrease in the yield of volatile oil extraction, and an increase in the number of distinctive and exclusive components.
In all EO samples, oxygenated monoterpenes and oxygenated sesquiterpenes were present in higher concentrations than their hydrocarbon counterparts. The highest level of monoterpenes was recorded in June, contrasting with the lowest amount observed in August. Conversely, the highest level of sesquiterpenes was recorded in August, while the lowest amount was observed in October. The content of oxygenated monoterpenes and sesquiterpenes in October and August (11.55% and 63.44%, respectively) was higher than in any other collection months (Table 1). It's important to note that oxygenated terpenoids, known for their contributions to fragrance and therapeutic properties, are considered essential markers for assessing the quality of EOs (24).
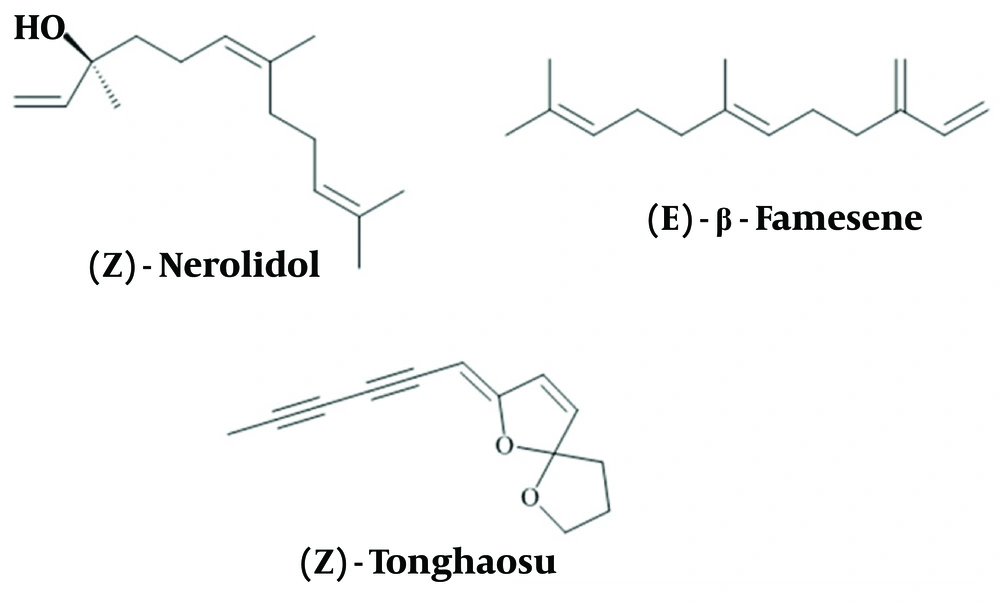
Throughout the four different harvesting times, the major constituents of A. biennis EO were (Z)-nerolidol, (E)-β-farnesene, and (Z)-tonghaosu (Figure 2). However, there were variations in the percentages of these constituents at each month. The percentage of (Z)-nerolidol showed only a slight increase from June to July (41.69% and 41.72%, respectively). In August, the proportion of (Z)-nerolidol significantly increased to 54.4%, before dropping to its lowest level in October (22.62%). The level of (E)-β-farnesene was recorded at 14.13% during the early vegetative stage, experienced a distinct decrease in July (6.89%), and then increased again during the full-flowering and late vegetative phases (11.49% and 16.38%, respectively). (Z)-tonghaosu exhibited its lowest value during the early vegetative stage (12.33%), followed by a rapid increase during the pre-flowering stage (18.61%), reaching its highest proportion compared to other growth stages.
Previous research by Lopes-Lutz et al. (10) identified (E)-β-farnesene and (Z)-β-ocimene as the major components in A. biennis EO. Another study by Nematollahi et al. (9) found camphor (24.6%), Artemisia ketone (11.4%), and α-pinene (10.2%) as the main components of A. biennis EOs. The diversity in extracted secondary metabolites can be attributed to various environmental factors such as the collection season, plant age, environmental conditions, and genetic factors (25, 26).
Investigating the effects of four different harvest times allowed for the first-time study of EO changes in A. biennis in Iran. This study revealed a distinctive and exclusive array of compounds in A. biennis EO compared to previous research (9, 10). The majority of exclusive compounds in A. biennis EO were attributed to the June and October harvests. Seven compounds were unique to June, including dihydrocarveol (0.83%), cabreuva oxide A (0.20%), alloaromadendrene (0.81%), β-humulene (0.37%), α-himachalene (0.68%), valerenol (0.56%), and lyratyl acetate (0.54%). Similarly, seven distinctive compounds were identified in the October harvest: O-cymene (0.18%), β-costol (0.73%), (E, E)-farnesol (0.9%), (Z)-caryophyllene (0.33%), γ-gurjunene (6.52%), ascaridole (1.50%), and valencene (0.81%).
The EO harvested in July contained six distinctive compounds, accounting for 10.94% of the total composition. These included cyclocopacamphenol (0.85%), (Z)-α-farnesene (1.18%), α-calacorene (1.19%), γ-costol (0.76%), β-bisabolene (0.91%), and viridiflorol (6.05%). During the full-flowering stage, which had the highest EO yield, two exclusive components with relatively high proportions were identified: E-α-necrodol (3.42%) and intermedeol (6.00%).
Nerolidol, which was found in the highest proportion in the EOs of the current study, is a sesquiterpene alcohol characterized by a floral odor and is a prominent component extracted from various medicinal plants. The wide-ranging pharmacological activities of nerolidol have attracted significant research interest, including its reported efficacy in anticancer and antitumor effects (27, 28), anti-inflammatory properties (29, 30), antiulcer activity (31), antimalarial activity (32), antifungal and antibacterial activity (33-36), anti-biofilm properties (37), antioxidant benefits (38), anti-parasitic effects (39, 40), and its role as a skin-penetration enhancer (41).
(E)-β-farnesene, a linear sesquiterpenoid, is another important component of the plant's essential oil. Farnesene is known for a wide range of biological effects, including antioxidant (42), antibacterial (43), and antifungal properties (44, 45). Additionally, farnesene has been employed as an intermediary in the synthesis of isophytol, the precursor of vitamin E (46).
4.2. Cytotoxicity Results
Colorectal cancer is the third most common cancer worldwide, ranking third among men and second among women. Additionally, breast cancer is the most common cancer in women in 157 out of 185 countries as of 2022. Notably, recent studies have shown that female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases (11.7%), followed by lung (11.4%) and colorectal (10.0%) cancers (47). Given this context, the cytotoxic effect of A. biennis EOs on breast and colon human cancer cell lines was investigated.
In a study conducted by Tayarani-Najaran et al. (48), the cytotoxic and apoptotic effects of the dichloromethane extract of A. biennis on the K562 and HL-60 cancer cell lines were examined. The A. biennis extract was found to induce apoptosis in human leukemia cells through a mitochondria- and caspase-dependent pathway.
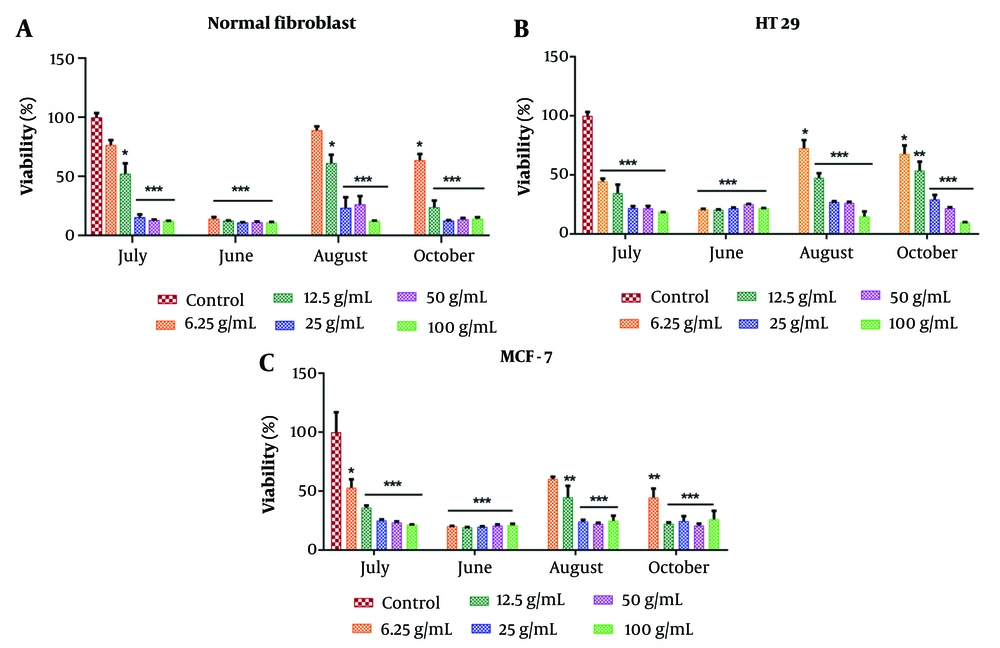
The percentage of cell viability after 24 hours of exposure to different concentrations of the EOs (0, 6.25, 12.5, 25, 50, and 100 μg/mL) was evaluated using the MTT method, and the results were analyzed with Prism software (Figure 3). The cytotoxicity effects on cell lines were found to be dose-dependent. According to Table 2, the most significant reduction in cell viability across all concentrations was observed with the EO extracted in June, demonstrating IC50 values of 2.99 ± 0.23, 2.41 ± 0.2, and 2.84 ± 0.15 μg/mL against normal fibroblast, HT-29, and MCF-7 cells, respectively (*P < 0.05).
The growth inhibition activity of various concentrations of Artemisia biennis essential oils (EOs) on human normal fibroblast, HT29, and MCF-7 cancer cell lines. Values were mean ± SEM of at least three independent experiments (n = 3). * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to the control group.
| Variables | June | July | August | October |
|---|---|---|---|---|
| Normal fibroblast | 2.99 ± 0.23 | 12.21 ± 0.38 | 14.96 ± 0.51 | 7.81 ± 0.36 |
| HT-29 | 2.41 ± 0.2 | 5.64 ± 0.57 | 11.46 ± 0.32 | 14.12 ± 0.81 |
| MCF-7 | 2.84 ± 0.15 | 7.66 ± 0.42 | 10.5 ± 0.41 | 5.5 ± 0.28 |
Generally, the compounds obtained from EOs in June and August had the highest and lowest proportions of monoterpenes, respectively. These phytochemicals have been extensively studied for their potential as anticancer agents in various tumor cell lines (49, 50), primarily by triggering apoptosis through intrinsic mechanisms, which result from oxidative stress induced by elevated ROS levels (51). In addition, monoterpenes have demonstrated cytostatic effects by blocking the cell cycle and inhibiting cell invasion and migration (51).
There are numerous reports on the cytotoxic effects of sesquiterpenes. For example, nerolidol—the main component of the EOs in the current study—suppresses the growth of bone cancer cells (MG-63) and induces apoptosis via PI3K/JNK regulation through cell cycle arrest (52). Many studies have investigated the anticancer potential of nerolidol (53, 54), particularly its effects on inhibiting cell growth and reducing cell proliferation. Lu et al. (55) suggested that nerolidol suppressed the growth of Glioblastoma multiforme cells and triggered cell death by modulating the activities of cell-cycle proteins through the p38 mitogen-activated protein kinase signaling pathways.
Additionally, (Z)-β-farnesene has shown significant anticancer potential, as reported by Afoulous et al. (56). Shaaban et al. (57) demonstrated that Matricaria chamomilla extracts could inhibit Caco-2 colon cancer cell migration, with tonghaosu identified as a key component responsible for this activity.
4.3. Conclusions
This study provided a quantitative and qualitative investigation of A. biennis EOs across different growth stages. Regardless of the growth stage, (Z)-nerolidol, (E)-β-farnesene, and (Z)-tonghaosu were identified as the major components of the EOs. The EO yields increased as the plant progressed to the full-flowering stage, with a subsequent decrease in the late vegetative stage. Based on the MTT assay results, the EO extracted during the early vegetative stage exhibited more potent cytotoxic activity against MCF-7 and HT-29 cells. The IC50 values for the normal human fibroblast and MCF-7 cell lines increased as the plant advanced to the full-flowering stage, but this trend reversed in the late vegetative stage. Interestingly, the content of oxygenated sesquiterpenes followed the same rise-and-fall pattern as the cytotoxic activity, while the trend for oxygenated monoterpenes exhibited the opposite pattern.