1. Background
Infectious agents such as fungi, bacteria, or harmful toxins stimulate the immune system to produce an inflammatory response to protect the body (1). However, when this inflammatory process goes beyond balance, it can cause several diseases, including cardiopathy, neuroinflammation, diabetes, and sepsis (2).
Zymosan is a pro-inflammatory agent extracted from the cell wall of Saccharomyces cerevisiae (3). Structurally, zymosan is a glucan that binds to glucose (4). In the innate immune system, zymosan is recognized by the Dectin-1 receptor, leading to an inflammatory process that increases tumor necrosis factor (TNF)-α, interleukin (IL-6), IL-12p40, and reactive oxygen species (ROS) production (3). This can lead to several inflammatory diseases, neurological diseases, cardiovascular issues, and metabolic disorders. Moreover, zymosan has been demonstrated to mediate severe acute and chronic inflammation, followed by shock and multiple organ dysfunction syndrome (MODS) (5, 6).
Perilla frutescens is an herb used as medicine and food, with beneficial parts including stems, leaves, and seeds (7). The juice of P.frutescens leaves is used to treat snake toxicity, ulcers, cough, and diabetes in Chinese medicine (8). This medicinal plant exhibits a wide range of biological activities, such as anti-inflammation, anti-cancer, anti-obesity, antioxidant, anti-osteoporosis, anti-ulcer, and other activities (8). Studies have reported that UA was isolated from the leaves of P.frutescens (8). Specifically, UA has many important pharmacological properties and may be involved in several signaling pathways to inhibit the development of chronic diseases (9). Ursolic acid has many beneficial effects as it may be involved in various cellular mechanisms such as nuclear factor-kappa B (NF-κB), apoptosis (10), insulin signaling (11), antioxidants in the brain (12), oxidative levels in the liver (13, 14), and atrophy (14).
2. Objectives
The role of UA in zymosan-induced inflammatory responses has not been studied. Therefore, this study demonstrated that UA from Vietnamese P.frutescens (L.) Britt. leaves inhibited zymosan-induced inflammation in macrophages.
3. Methods
3.1. Plant Material
The P.frutescens (L.) Britt. leaves were collected from Thai Binh, Vietnam, in July 2018. The samples were identified (HNU024060) by the Biological Museum, VNU, University of Science.
3.2. Chemicals
Zymosan, UA, laminarin (Dectin-1 antagonist), polysaccharide galactan, anisaldehyde solution, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich. tumor necrosis factor-α, IL-6, and IL-12p40 ELISA kits were purchased from BD Pharmingen (Franklin Lakes, NJ). Specific antibodies against extracellular signal-regulated kinases (ERK)1/2, phospho-(Thr202/Tyr204)-ERK1/2, p38, phospho-(Thr180/Tyr182)-p38 were purchased from Cell Signaling Technology (Beverly, MA). Phospho-(Ser345)-p47phox was purchased from Sigma-Aldrich, and p47phox was purchased from Thermo Fisher Scientific. Ethanol, n-Hexane, methanol, ethyl acetate, and phosphate buffer were purchased from Merck.
3.3. Extraction and Isolation
Ursolic acid was extracted from P.frutescens leaves according to the method previously described by Huaman et al. (15). Specifically, P.frutescens leaves (1000 grams) were collected, dried, and pulverized into powder (108 grams). The dry powder was then soaked in ethanol (96%) (10 grams in 100 mL). The organic phase was renewed twice every 24 hours. The extract was then evaporated to obtain the residue (9.68 grams). The residue was collected and defatted with hexane (180 mL). The organic phase was removed, and the residue (5.84 grams) was collected and redissolved in ethanol (400 mL) before being decolorized with activated carbon. Finally, ethanol (96%) (250 mL) solution was used to crystallize until a white amorphous solid (2.87 grams) of UA was obtained.
3.3.1. Thin-Layer Chromatography Analysis of Ursolic Acid
The presence of UA was indicated by thin-layer chromatography (TLC) with a mobile phase of n-hexane and ethyl acetate (3:1 v/v), detected by anisaldehyde solution (16).
3.3.2. High-Performance Liquid Chromatography Analysis of Ursolic Acid
The purity of UA was evaluated by the high-performance liquid chromatography (HPLC) method using a Shimadzu system. The mobile phase used a solvent system of 0.1 M phosphate buffer and methanol (pH = 3) (10:90 v/v). The experiment was performed at 21°C, with a sample volume of 10 μL, a flow rate of 0.9 mL/min, and a detection wavelength of 210 nm (17).
3.4. Cell Culture
Raw 264.7 cells were obtained from the American Type Culture Collection and cultured according to the biobank instructions to achieve 80% - 85% confluence. Briefly, the cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10% FBS, sodium pyruvate, non-essential amino acids, penicillin G (100 IU/mL), and streptomycin (100 µg/mL) at 37°C with 95% humidity and 5% CO2.
3.5. Assay of Cell Viability
Cell Counting Kit-8 (CCK, Dojindo Laboratories, Kumamoto, Japan) was used for evaluating cell viability. 1×10^6 cells/well were incubated with UA (10 - 40 μg/mL) for 48 hours. Then, 10 μL of CCK was added to the cells for 1 hour. The absorbance of the cells was measured at 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, San Jose, CA, USA).
3.6. Enzyme-Linked Immunosorbent Assay
Ursolic acid was added to the cells for 45 minutes before stimulation with zymosan. Enzyme-linked immunosorbent assay reagents were used to analyze the levels of TNF-α, IL-6, IL-10, and IL-12p40 in the supernatants after 18 hours.
3.7. Western Blotting
The phosphorylation of ERK1/2, p38, Ser345, and p47phox after treatment was detected by Western blotting (18). Raw 264.7 cells were incubated with UA after treatment with laminarin, polysaccharide galactan, or 0.1% DMSO. Then, zymosan (100 µg/mL) was added to the cells. Afterward, the cells were lysed. Primary antibodies were used against the following proteins: Extracellular signal-regulated kinases1/2 (1:1000), phospho-(Thr202/Tyr204)-ERK1/2 (1:1000), phospho-(Thr180/Tyr182)-p38 (1:1000), p38 (1:1000), phospho-(Ser345)-p47phox (1:1000), and p47phox (1:1000). A secondary antibody, HRP-linked anti-rabbit antibodies (cell signaling technology), was used. Chemiluminescence assay (ECL; Amersham-Pharmacia) was used for the development of the membranes.
3.8. Measurement of Intracellular Reactive Oxygen Species
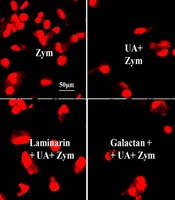
The measurement of intracellular superoxide levels was described previously (18). The cells were incubated with UA for 45 minutes after treatment with laminarin (0.25 mg/mL), polysaccharide galactan (0.25 mg/mL), or 0.1% DMSO for 60 minutes. Then, zymosan (100 µg/mL) was added to the cells for 30 minutes. In the next step, 2 μM DHE (Calbiochem) was incubated with the cells for 15 minutes at 37°C in CO2. A laser scanning confocal microscope (LSM 510) and a Carl Zeiss vision system (LSM510) were used to analyze the cells and the average relative fluorescence intensity of each group.
3.9. Determination of Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity
Raw 264.7 cells were incubated with UA for 45 minutes after treatment with laminarin (0.25 mg/mL), polysaccharide galactan (0.25 mg/mL), or 0.1% DMSO for 60 minutes. Then, zymosan (100 µg/mL) was added to the cells for 30 minutes. Lucigenin chemiluminescence assay (Sigma) was used to evaluate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activities in the cells as described previously (18).
3.10. Statistical Analyses
The results from the independent experiments are expressed as the mean ± SD. The data were analyzed using Student’s t-test with ANOVA to determine the differences among the groups. P < 0.05 was considered significant.
4. Results
4.1. Thin Layer Chromatography Analysis and High-Performance Liquid Chromatography Analysis of Ursolic Acid
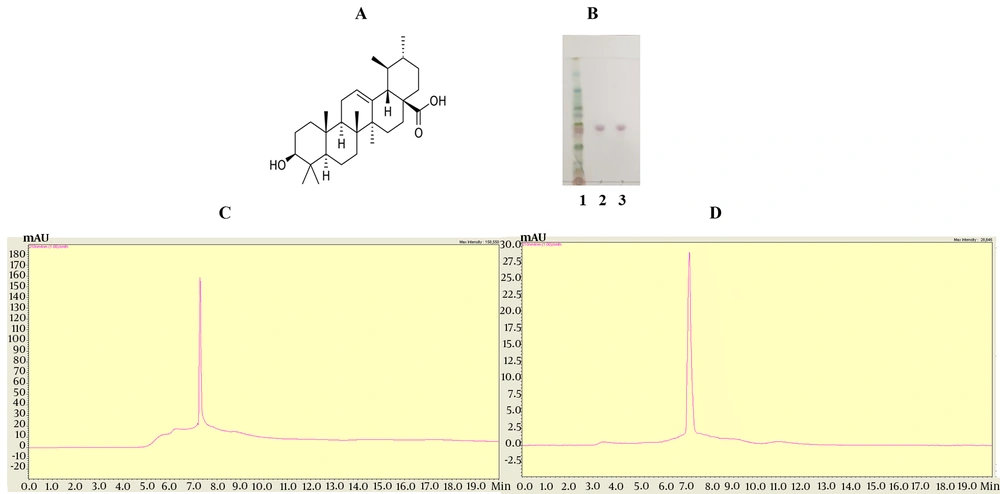
The struture of UA (Figure 1A). Thin layer chromatography analysis showed that the purified UA sample exhibited a single band equivalent to the standard UA (Figure 1B). The sample UA was further tested for purity using HPLC. The results indicated that the sample UA was consistent with the standard UA in both shape and retention time (Figure 1C, D).
4.2. Cytotoxicity of Ursolic Acid to Cell Viability
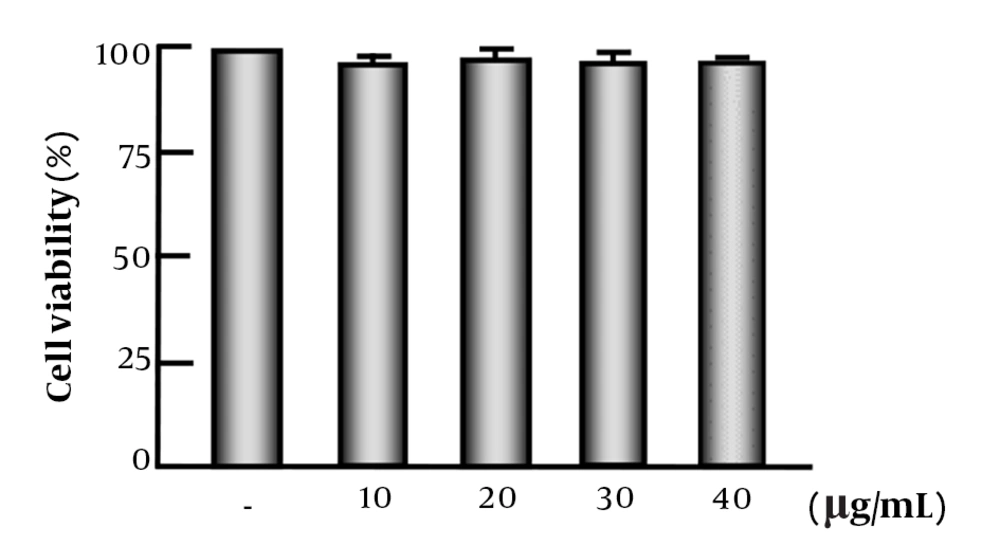
The results showed that UA levels between 10 and 40 μg/mL did not affect cell viability (Figure 2).
4.3. Ursolic Acid Inhibits Cytokine Production by Zymosan-Activated Macrophages
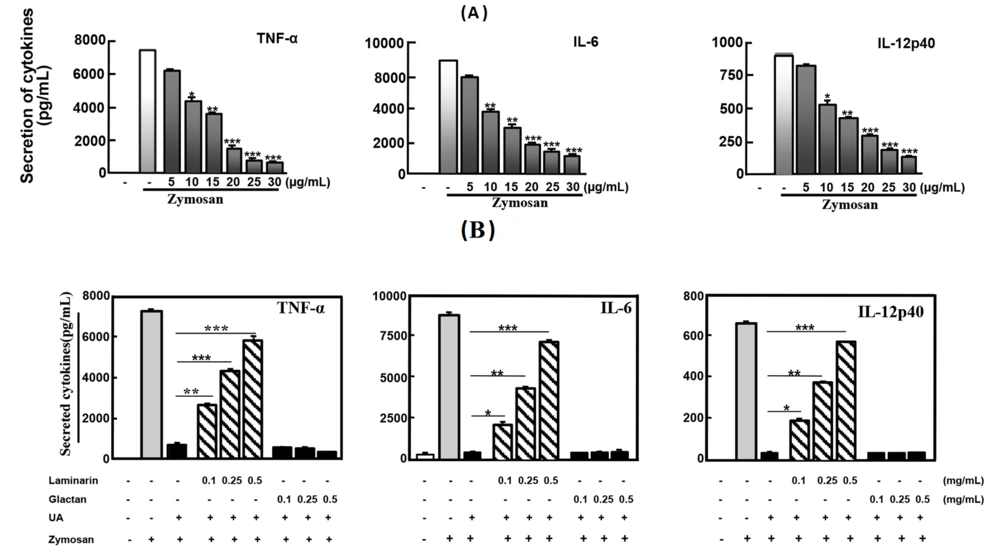
The anti-inflammatory effects of UA were examined for the production of pro-inflammatory cytokines. Ursolic acid at 30 μg/mL (P < 0.001) strongly inhibited TNF-α, IL-6, and IL-12p40 in Raw 264.7 cells (Figure 3A).
Ursolic acid inhibited the zymosan-induced inflammatory responses through Dectin-1. A, Raw 264.7 were treated with UA at concentrations (5, 10, 15, 20, 25, 30 µg/mL) before stimulation with zymosan (100 µg/mL); B, Raw 264.7 was incubated UA (30 µg/mL) for 45 min after pre-treated with laminarin, polysaccharide galactan or 0.1% DMSO for 60 min. Then, zymosan (100 µg/mL) was added in the cell for 18h and supernatants were harvested for enzyme-linked immunosorbent assay (ELISA). The figure is presented as mean ± SD of five experiments. ***P < 0.001 compared to control culture group. -, 0.1% dimethyl sulfoxide (DMSO).
4.4. Ursolic Acid Modulates Dectin-1-Mediated Cytokine Production in Response to Zymosan
The production of zymosan-induced cytokines was significantly increased in Raw 264.7 cells pretreated with laminarin (Figure 3B). These data indicated that UA inhibited pro-inflammatory cytokine production in Raw 264.7 cells through Dectin-1.
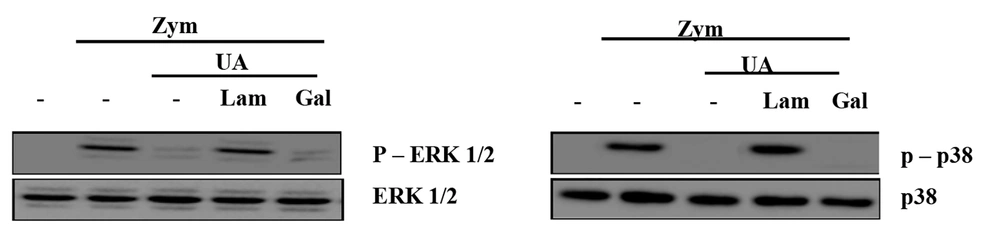
4.5. Ursolic Acid Modulates Dectin-1-Mediated Mitogen-Activated Protein Kinase Activation in Recognizing to Zymosan
The phosphorylation of ERK1/2 and p38 induced by zymosan was detected in Raw 264.7 cells pretreated with laminarin, which blocks Dectin-1. In contrast, phospho-p38 and phospho-ERK1/2 were not detected in cells that were not stimulated in the presence of laminarin (Figure 4). The data indicated that UA significantly inhibited the activation of MAPK in Raw 264.7 cells in response to Dectin-1/zymosan.
Ursolic acid inhibited the zymosan-induced mitogen-activated protein kinase (MAPK) activation through Dectin-1. Raw 264.7 was incubated UA (30 µg/mL) for 45 min after treating with laminarin (0.25 mg/mL), polysaccharide galactan (0.25 mg/mL) or 0.1% dimethyl sulfoxide (DMSO) for 60 min. Then, zymosan (100 µg/mL) was added in the cell for 30 min. Western blot analysis detects the activation of p38 and extracellular signal-regulated kinases (ESK)1/2. -, 0.1% DMSO; UA, ursolic acid; Zym, zymosan; Lam, laminarin; Gal, galactan.
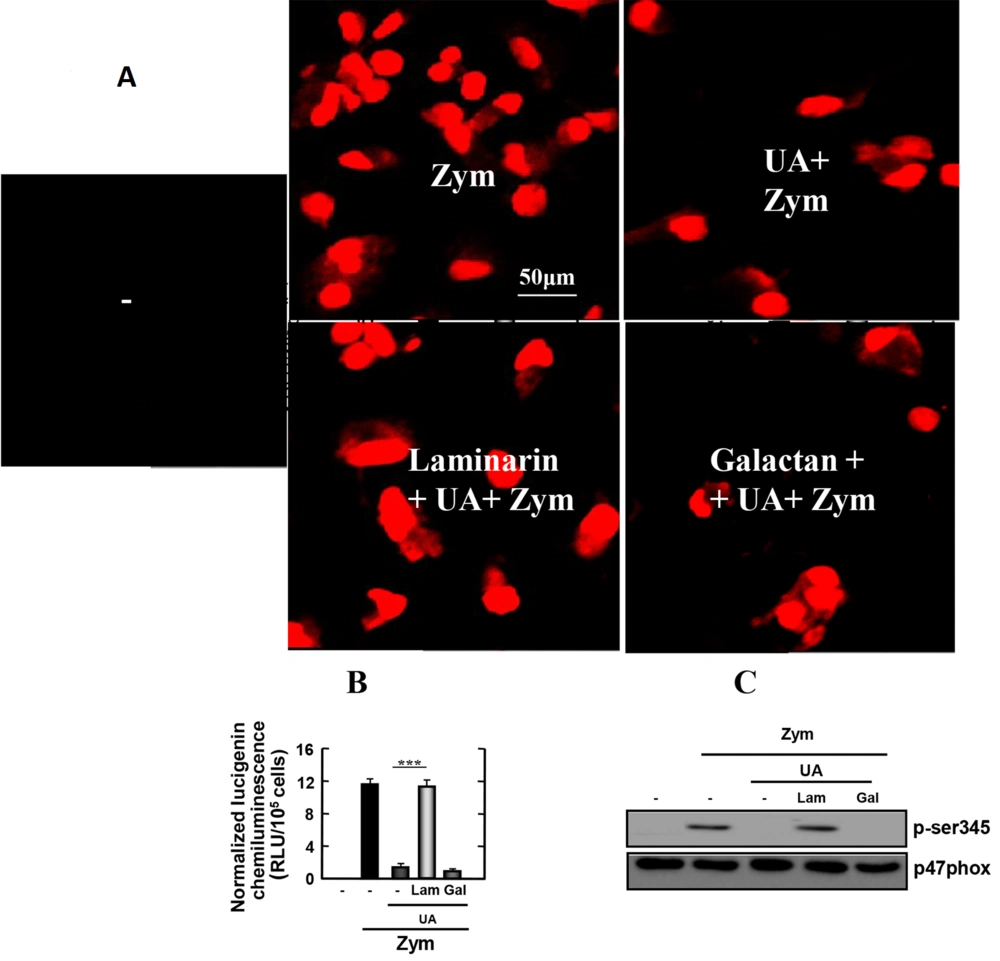
4.6. Ursolic Acid Inhibits Reactive Oxygen Species
Because Dectin-1-mediated uptake of zymosan generates reactive oxygen species (ROS), we investigated whether UA affects Dectin-1-mediated ROS production in macrophages infected with zymosan. As shown in Figure 5A, zymosan strongly induced superoxide production in macrophages. However, zymosan-induced superoxide secretion in stimulated macrophages was significantly blocked by UA. As expected, superoxide production increased in response to zymosan in macrophages stimulated with laminarin and UA. The findings indicated that UA regulates ROS through Dectin-1 in macrophages infected with zymosan.
Reactive oxygen species (ROS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activities by zymosan-induced in Raw 264.7 cells are inhibited by Ursolic acid (UA). Raw 264.7 was incubated UA (30 µg/mL) for 45 min after treating with laminarin (0.25 mg/mL), polysaccharide galactan (0.25 mg/mL) or 0.1% dimethyl sulfoxide (DMSO) for 60 min. Then zymosan (100 µg/mL) were added in the cell for 30 min (for A, B) and 15 min (for C). Ursolic acid inhibits zymosan-induced ROS generation (for A) and NADPH oxidase activities (for B) in cells. The cells were lysed and analyzed by western blot (for C). The figure is presented as the mean ± SD of three experiments (***, P < 0.001 compared to control culture group). -, 0.1% DMSO; UA, ursolic acid; Zym, zymosan; Lam, laminarin; Gal, galactan.
4.7. Ursolic Acid Blocked Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity
Nicotinamide adenine dinucleotide phosphate oxidase is the enzyme in leukocytes responsible for ROS production. Therefore, we explored the role of UA in NADPH oxidase activity in macrophages stimulated with zymosan. The results are shown in Figure 5B. Macrophages stimulated with zymosan induced a high level of NADPH-induced lucigenin chemiluminescence. In contrast, macrophages stimulated with UA, in the presence or absence of galactan, showed abrogated NADPH oxidase activity. As expected, macrophages induced a high level of NADPH-induced lucigenin chemiluminescence in the presence of laminarin. This demonstrated that the effect is specifically due to Dectin-1 receptor activation.
Nicotinamide adenine dinucleotide phosphate oxidase is activated by the phosphorylation of p47phox. Therefore, we examined the levels of zymosan-induced p47phox phosphorylation in macrophages. As shown in Figure 5C, the zymosan-induced phosphorylation of p47phox was detected in macrophages pretreated with laminarin, which blocks Dectin-1. In contrast, phosphorylation of p47phox was not detected in cells that were not stimulated in the presence of laminarin. These data suggest that UA suppresses the production of NADPH oxidase through Dectin-1 in macrophages infected with zymosan.
5. Discussion
Inflammation is an important protective mechanism for health because it is the immune system's response to harmful stimuli (1). However, uncontrolled acute inflammation can become chronic, contributing to many chronic inflammatory diseases (2). Therefore, new strategies to inhibit inflammation that will help improve patient survival are very important. Ursolic acid can inhibit tumor invasion, chromosomal aberration, and tumorigenesis, and it can suppress NF-κB signaling in cancer cells (10). Ursolic acid has also been demonstrated to protect the liver by reducing apoptotic signaling and oxidants (13). Additionally, UA can decrease inflammation and the expression of markers of cardiac damage (19). However, the role of UA in the regulation of zymosan-dependent signaling is unclear. The current data show that UA plays an important role in regulating zymosan-stimulated inflammatory responses.
The results of our study show that UA regulates zymosan-induced inflammatory cytokine secretion in macrophages, and this depends on Dectin-1. Recent research also showed that UA inhibited LPS-induced prostaglandin E2, iNOS, cyclooxygenase, and ROS in macrophages (20, 21). The inflammatory signaling of acute and chronic inflammation in immune cells is mediated by the mitogen-activated protein kinase (MAPK) pathway (22). The data from this study show that UA inhibits zymosan-stimulated MAPK activation through the Dectin-1 receptor. This is consistent with previous research in which UA inhibited the phosphorylation of p38 and ERK1/2 in SK-MEL-24 cells (21).
Dectin-1 signaling triggers innate immune responses leading to the production of inflammatory factors such as ROS (23, 24). In this study, we show that ROS, NADPH oxidase activity, and the phosphorylation of p47phox in macrophages were suppressed by UA, depending on Dectin-1. Reactive oxygen species is critically involved in acute and chronic inflammation, inducing necrosis through DNA single-strand breaks (25). More importantly, the inhibition of Ser345 phosphorylation of p47phox suppressed ROS production in synovial neutrophils in patients with rheumatoid arthritis (26). Together with previous studies, our data show the potential for using UA to manage acute inflammation through ROS regulation.
To date, many studies have shown that UA plays a very important role in controlling diseases, including inflammatory and infectious diseases (9). The data in this study first demonstrate a role for UA in the regulation of zymosan-dependent signaling. For many years, glucocorticoids have been considered an important component in the treatment of inflammatory diseases because of their strong anti-inflammatory properties (27). However, the use of glucocorticoids in clinical practice is still limited due to a number of side effects related to high concentrations, dosage, and long-term use (26). Therefore, novel anti-inflammatory approaches based on glucocorticoid mechanisms could lead to the development of new anti-inflammatory drugs that relieve inflammation with less toxicity and fewer side effects. As zymosan is a strong agent for inducing systemic inflammatory response (4), the data in this study suggest a therapeutic role for UA in fungus-related diseases and inflammatory conditions.
5.1. Conclusions
This study demonstrates that UA markedly inhibits zymosan-stimulated pro-inflammatory factors such as pro-inflammatory cytokines, MAPKs, ROS, NADPH oxidase, and the phosphorylation of p47phox in macrophages through the Dectin-1 receptor. Therefore, the data in this study may suggest new opportunities and therapeutic approaches for treating zymosan-induced inflammation.