1. Background
In recent years, drug delivery approaches have received extensive attention in pharmaceutical science. From this perspective, drug administration through controlled delivery systems is a favorable topic (1-3). These systems maintain the drug concentration at intended levels for a longer period, subsequently decreasing the dosing intervals. Moreover, conventional approaches are usually associated with adverse effects and treatment failure, leading to an increased demand for new drug carriers (4-7). Recently, spectacular advances in nanomaterials have been noticed in the field of drug delivery due to their unique properties, such as a high surface-to-volume ratio, customizable particle size, and high solubility. As a class of nanocomposites, layered double hydroxide (LDH) nanoparticles, also known as anionic clays or hydrotalcite-like systems, have shown the capacity to preserve drug molecules in the interlayer spaces (8, 9). Originally derived from the structure of brucite (Mg (OH)2), these layered nanostructures can be formed by replacing a part of divalent cations with trivalent cations, typically stabilized by exchangeable interlayer anionic species (either inorganic or organic) and water molecules. Regarding the size/charge ratio of anions and the dimensions of guest species, there should be a host-guest relationship between them. In this context, LDH nanoparticles can be easily manipulated for intercalating various drug molecules with many metal-anion combinations and possible compositions to reduce the degradation rate and provide a sustained release profile (10, 11).
By incorporating a polymer into LDH nanoparticles, a polymer-based nanocomposite film can be obtained with unique functions and structures, which can be defined as solution intercalation, melt intercalation, or emulsion intercalation through a layer-by-layer self-assembly method (12, 13). In the field of drug delivery, the utilization of inorganic materials may be associated with the production of harmful byproducts and environmental impacts. Instead, using natural substances and green synthesizing methodologies can minimize energy consumption and pollutant production, as well as improve human health (14, 15). Accordingly, natural-derived substances (such as honey) that structurally consist of several organic macromolecules (like carbohydrates, proteins, nucleic acids, and fatty acids) are drawing interest (16, 17). Natural-based nanocomposite materials are generally considered non-toxic and biocompatible, with high chemical stability and pH-dependent solubility (12, 18). They are prepared through inexpensive processes and can be easily modified with unique physicochemical properties for different applications such as environmental science, catalysis, biosensing, cosmetics, and medicine (10, 19). Although switching to biological sources might address numerous significant problems, the possibility of rapid degradation of active ingredients during storage, either by hydrolysis or oxidation, and inadequate therapeutic responses due to restricted release profiles highlight the necessity of introducing drug delivery systems that can address these issues (20).
2. Objectives
Therefore, the current work was proposed to introduce a new drug delivery platform that can preserve active ingredients from rapid degradation and provide a prolonged release profile by using natural-derived substances. Accordingly, we aimed to green synthesize and evaluate the physicochemical characteristics of a natural-based nanocomposite using honey as an interfacial ligand, named honey-incorporated Cu/Al-LDH (Ho@Cu/Al-LDH) nanocomposite. In this respect, ibuprofen (Ibu; a poorly water-soluble drug prone to immediate degradation in extreme environments) was chosen as a model pharmaceutical agent to investigate the potential applications of this system for controlled drug delivery. Further, the dye removal potential of this nanocomposite was also assessed as a low-cost and recyclable adsorbent.
3. Methods
3.1. Materials
Cupric nitrate trihydrate (Cu(NO3)2·3H2O), aluminum nitrate nonahydrate (Al(NO3)3·9H2O), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), ascorbic acid, sodium acetate (CH3COONa), acetic acid (CH3COOH), ferric chloride (FeCl36H2O), ferrous sulfate (FeSO47H2O), methylene blue, and Ibu (98% purity) were purchased from Sigma-Aldrich, Germany. Honey was derived from Lalezar, Kerman province, Iran. Sodium hydroxide (NaOH), hydrochloric acid (HCl), and ethanol (96%) were purchased from Merck, Germany.
3.2. Preparation of Ho@Cu/Al- Layered Double Hydroxide Nanocomposite
The Cu/Al-LDH nanoparticles were prepared using honey as an anionic ligand based on previous literature by a co-precipitation-assisted hydrothermal method (21-23). Briefly, 2.5% w/v of Cu(NO3)2· 3H2O and Al(NO3)3·9H2O in a 2:1 molar ratio were dissolved in 20 mL of a water/ethanol (1:1) solution. To increase the pH of the solution to 9.5, NaOH (2 M) was added along with magnetic stirring (400 rpm for 2 hours at 50°C). Subsequently, the solution was mixed with 5% w/v of honey, and stirring continued for a further 4 hours under an argon atmosphere. It was then poured into the autoclave and heated for 8 hours at 110°C. Afterward, the product was collected and dried at 80°C overnight, followed by several washing steps with water/ethanol solution to remove excess anions and obtain the Ho@Cu/Al-LDH nanocomposite (18, 21, 24). However, no attention was paid to control other physicochemical properties.
3.3. Physicochemical Characterization
Following the preparation of the Ho@Cu/Al-LDH nanocomposite, physicochemical properties were assessed. High-performance thin-layer chromatography (HPTLC) was employed to confirm the presence of honey in the product using water/ethanol (1:1) solution and Silica gel 60 F254 HPTLC aluminum plates (10 × 10 cm; Merck, Germany) as the mobile and stationary phases, respectively (25-27). Following the advancement in a developing chamber, densitometry analysis (CAMAG TLC Scanner 3, Switzerland) was used to quantify the bands at 254 nm using WIN CATS software (version 4 X) (28). The physical stability was also assessed using centrifugation durability and thermodynamic accelerated tests (heating-cooling and freeze-thaw cycles), while visually observing for any changes (29). The dynamic light scattering (DLS) method (VASCO®, Cordouan Technologies, France) was used to determine the average particle size and polydispersity index (PDI). It is a rapid and non-destructive method based on Brownian motion in the liquid phase (30, 31). Additionally, the morphology and uniformity of the prepared nanocomposite were assessed using scanning electron microscopy (SEM) analysis with an acceleration voltage of 15 kV (FE-SEM, MIRA 3 XM, Tescan Inc., USA) according to previously reported literature (32, 33).
3.4. Study of Dye Removal Potential
Based on previously reported literature, the potential dye removal applications of the prepared nanocomposite were also assessed (23, 33, 34). Accordingly, the sample suspension (dispersed in water/ethanol (1:1) solution) was mixed with a methylene blue diluted solution (100 ppm) at room temperature with continuous stirring (150 rpm for 2 hours). The liquid and solid phases were separated by filtering, and the quantity of methylene blue was then calculated at predetermined intervals using a UV spectrophotometer (λmax: 664 nm). The experiment was conducted in triplicate at two different pH values (7.4 and 9.5), and dye removal (%) was reported as mean ± SD.
3.5. Study of Total Antioxidant Capacity
The ferric reducing antioxidant power (FRAP) assay was conducted to evaluate the total antioxidant capacity of the Ho@Cu/Al-LDH nanocomposite with slight modifications (35-37). Accordingly, 200 μL of the sample suspension was mixed with 1800 μL of FRAP reagent, which contained 2.5 mL of TPTZ solution (5 mM of TPTZ in 20 mM HCl), 2.5 mL of FeCl3·6H2O solution (20 mM), and 25 mL of acetate buffer (300 mM, pH 3.6), followed by incubation in the dark (tubes covered with aluminum foil) for 10 minutes at 25°C. Using aqueous solutions (50 to 1000 μM) of ferrous sulfate heptahydrate (FeSO4⋅7H2O), the standard calibration curve was plotted at 593 nm. The experiment was conducted in triplicate during a 30-day assessment. Results were compared to ascorbic acid and reported as mean ± SD. Eventually, the FRAP value was determined as the micromolar equivalent of ferrous in the sample mass (μM Fe (II)/g). Theoretically, different concentrations of an antioxidant agent cause changes in the FRAP assay absorbance equivalent to Fe(II) solution (1 μM).
3.6. Study of Entrapment Efficiency and Drug Release
To evaluate the potential applications of this nanocomposite for controlled drug delivery, Ibu was chosen as a model pharmaceutical agent. Therefore, the entrapment efficiency (EE) of Ibu-loaded Ho@Cu/Al-LDH (Ibu-Ho@Cu/Al-LDH) nanocomposite was studied with reference to previously reported studies (22, 38, 39). Primarily, the calibration curve of Ibu was plotted at λmax (228 nm) and linearity was checked (R² = 0.996; LOD: 3.78 and LOQ: 12.56 ppm). Next, the sample suspension was mixed with Ibu solution (10 mg/mL) by continuous stirring (100 rpm) for 10 hours at room temperature. Afterward, 100 μL of the prepared nanocomposite was diluted with a water/ethanol solution (1:1), and the concentration of Ibu was measured using UV spectrophotometric analysis. The experiment was conducted in triplicate, and Ibu content (%) was reported as mean ± SD according to Equation 1:
Wt is the total amount of Ibu determined in the Ibu-Ho@Cu/Al-LDH nanocomposite, and Wi is the total quantity of Ibu used. To ensure there is no interference from other components, the unloaded formulation was used as a blank. The release profile of the prepared nanocomposite was assessed under sink conditions using a Franz diffusion cell with slight modifications (2, 21, 22). The cellophane membrane (synthetic semi-permeable membrane with a molecular weight cut-off of 12 kDa) was deposited in the receiving medium for complete saturation overnight. The formulation was poured into the donor compartment, and sampling was carried out under constant conditions (stirring at 200 rpm and 37 ± 1°C) at predetermined intervals. Subsequently, samples were subjected to UV spectrophotometry analysis at λmax. The release profile of the Ibu-Ho@Cu/Al-LDH nanocomposite was assessed under two different pH levels (7.4 and 9.5). Moreover, the release profile of pure Ibu (dissolved in water/ethanol) was also evaluated to provide a better concept. The experiment was conducted in triplicate, and results were expressed as mean ± SD.
Following, different kinetic models (zero-order, first-order, Higuchi, Hixon-Crowell, and Korsmeyer-Peppas) were mapped to define diffusion characteristics and the release profile. In this respect, the best-fitted equation was selected based on the R² value approaching and the slope of the respective plots. Further, the Akaike Information Criterion (AIC) was also used to test the goodness of fit according to the maximum likelihood using the KinetDS software program (KinetDS 3.0). This statistical measure is used to assess the applicability of models for a given set of data in terms of their predictive accuracy and relative performance (40, 41). When comparing several models, the one with the minimum AIC value is considered the best fit according to Equation 2:
Where n is the number of sampling intervals (release data points), p stands for the number of parameters (independent variables) in the model, and WSSR is the abbreviation for the weighted sum of squares of residues, estimated by Equation 3:
Aaccording, wi is an optional weighing factor, yi denotes observed value, and y'i stands for predicted value.
4. Results and Discussion
4.1. Physicochemical Characterization
The result of the HPTLC profile is illustrated in Figure 1. Accordingly, there is a high accordance between the yielding band of honey and the prepared nanocomposite, indicating the presence of ligands in this nanostructure. The HPTLC analysis can create a chromatographic fingerprint in the form of a unique sequence of peaks corresponding to the entire analyzed sample (28). Megalathan et al. (42) used thin layer chromatography (TLC) analysis to evaluate the intercalation of curcuminoids into Mg/Al LDH nanoparticles. Shi et al. (25) investigated the chelator-free labeling potential of Mg/Al-LDH nanoparticles coated by bovine serum albumin for different isotopes using TLC, as this method can determine the unbound samples. In another study, Gilanizadeh and Zeynizadeh used HPTLC analysis to assess the purity of substrates and products as well as to monitor the completion of synthesis reactions (26). Khashaba et al. synthesized Fe3O4/FeOOH magnetic nanocomposites for the extraction of triptan family members (such as zolmitriptan) using HPTLC determination in the presence of paracetamol and metoclopramide (43).
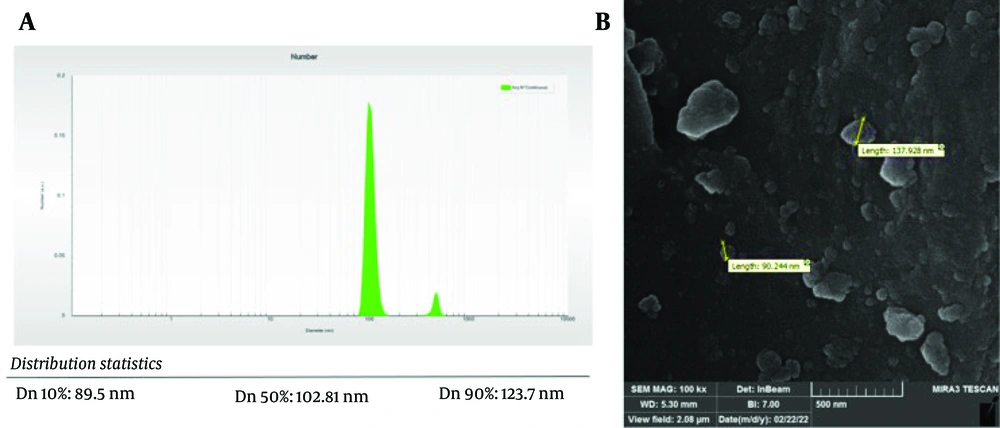
According to the thermodynamic accelerated stability tests, the prepared nanocomposite demonstrated acceptable physical stability. Visually, there was no sign of fickleness or inconsistency following the heating-cooling and freeze-thaw cycles. The structure also exhibited high durability in the centrifugation cycle, indicating good mechanical strength. Figure 2 shows the results of DLS analysis and SEM imaging. Based on the DLS results, the average particle size of Cu/Al-LDH nanoparticles was approximately 123.7 nm with a PDI of 0.37, indicating a high degree of uniformity. The PDI is a measure of the size distribution in a given sample. PDI values range from 0 to over 1, where a lower PDI value indicates higher homogeneity. Systems with a high degree of homogeneity are anticipated to have a greater predictive capability for physicochemical properties, stability during storage, and release parameters. Similar to the DLS data, SEM micrographs also indicated a restricted range for particle size distribution. However, the presence of some coarse particles in the product might emphasize the necessity of fine milling.
4.2. Study of Dye Removal Potential
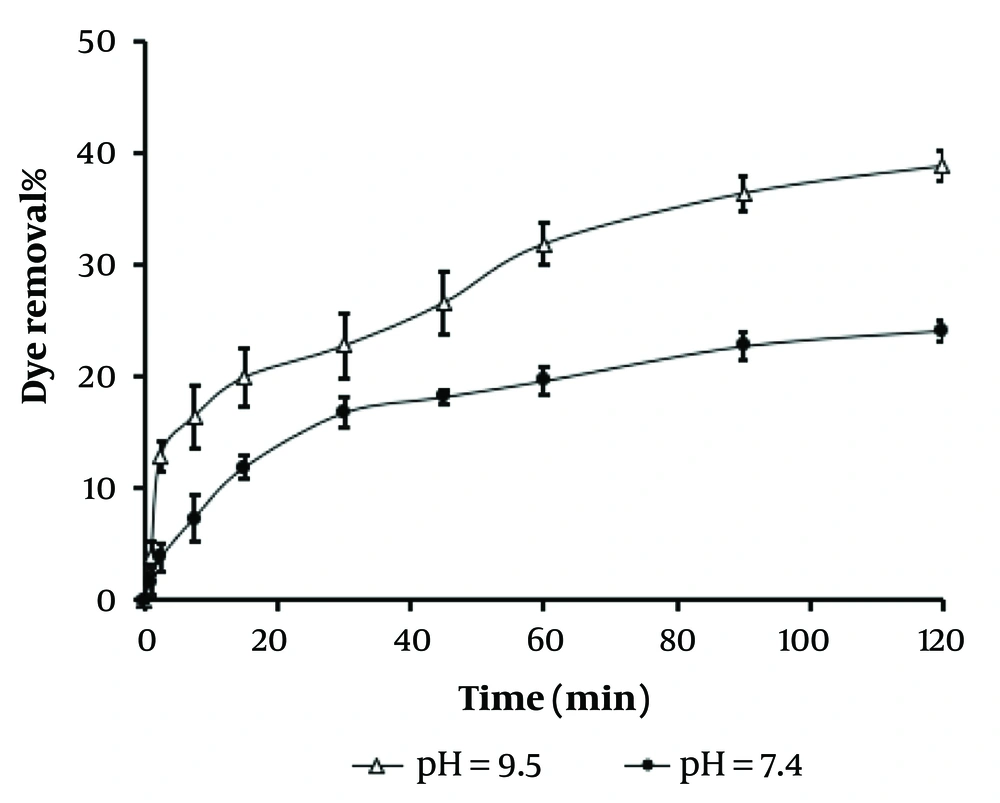
Figure 3 represents the results of the adsorbent profile and dye removal potential for methylene blue. Accordingly, an alkaline pH (9.5) provides a better adsorbent profile due to the higher presence of OH⁻ anions, as the LDH structure becomes positively rigid in alkaline conditions. Dye removal is an important process for industrial wastewater treatment, which can be achieved by several techniques, including chemical oxidation, membrane technology, coagulation, photocatalytic degradation, and physical adsorption. Among these, adsorption is considered the most effective technique due to its low cost, simple design, and high efficiency. This process might depend on several factors such as adsorbent amount, contact time, initial dye concentration, and pH (44, 45). So far, various adsorbents such as carbon-based materials, metal oxides, and polymers have been used to effectively remove pollutants. These adsorbents work by intercalating/capturing dye molecules and thus purifying the wastewater. Therefore, LDH nanocomposites might be of interest due to their multilamellar structure, abundant interlayer spaces, limited toxicity, facile synthesis, and high stability (46, 47).
Abdel-Hady et al. assessed the adsorption pattern and textural properties of Zn/Mg/Al-LDH toward crystal violet dye as a low-cost and recyclable adsorbent (23). Based on their study, the synthesized LDH platform shows a pH-sensitive behavior with higher dye removal in alkaline conditions. Other factors that positively affect dye adsorption include contact time, initial dye concentration, and the LDH dose. However, the excessive presence of LDH in the test environment can become an obstacle for the active sites, leading to aggregation of LDH layers and reduction of the adsorbent surface area. In another study, Yadav and Dasgupta evaluated the potential of Mg/Al-LDH for the adsorption of methyl orange dye from aqueous solutions (48). Accordingly, particles with a size range of 60 to 120 nm were synthesized by the co-precipitation method under a nitrogen atmosphere, while the pH and temperature of the dye solution had a major effect on the adsorption kinetics. de Sá et al. investigated the effects of pH, contact time, and dye concentration on the removal properties of the Ca/Al-LDH system for the adsorption of Sunset Yellow FCF food dye (a petroleum-derived orange azo dye) (49). Their results indicated that the pH range of 4.0 to 10 is effective for dye removal, as low pH modifies the surface charge of the adsorbent and potentially increases the degree of dye dissolution from interlayer spaces.
In summary, the present study indicates the possibility of using LDH nanocomposites to develop new adsorbents with elevated pollutant removal capacity for environmental protection.
4.3. Study of Total Antioxidant Capacity
Oxidation is one of the most common degradation pathways of pharmaceutical ingredients. Therefore, introducing drug delivery systems that can combat this challenge and preserve drug molecules from destructive factors is of interest. The FRAP technique exploits antioxidant agents in a redox-linked colorimetric assay, where a higher absorbance indicates a superior FRAP value and greater antioxidant capacity (37, 50). The FRAP value of this nanocomposite was measured to be 298.42 ± 0.93 and 286.37 ± 1.45 μM Fe (II)/g on the production day and after 30 days, respectively, showing minimal alteration. Therefore, this system not only can be a candidate for drug delivery but also can be used to preserve incorporated molecules from immediate degradation followed by oxidation. Fundamentally, the antioxidant capacity refers to the exploitation of honey in this system. Honey consists of several organic molecules (such as terpenoids, alkaloids, and flavonoids) with high antioxidant capacities (16, 51). Several studies have revealed the potential antioxidant properties of honey (52, 53). Neupane et al. studied the antioxidant and antimicrobial properties of iron oxide nanoparticles loaded with Himalayan honey (54). In another study, Keskin et al. synthesized silver nanoparticles based on chestnut honey and evaluated the physicochemical properties for use as a potential drug delivery system in fields such as medicine, pharmaceuticals, and cosmetics (55).
4.4. Study of Entrapment Efficiency and Drug Release
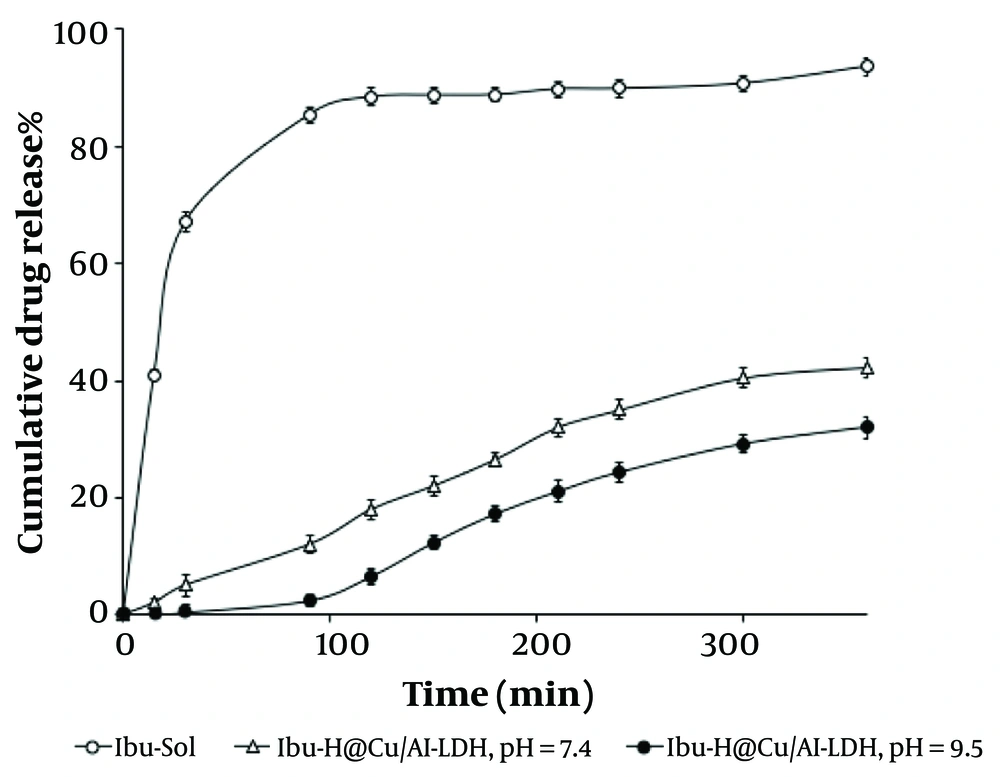
The EE of the Ibu-Ho@Cu/Al-LDH nanocomposite was measured to be 73.15 ± 0.401%, followed by 72.87 ± 0.547% within a 30-day assessment, revealing negligible alteration. In drug delivery systems, content uniformity and chemical stability of loaded ingredients are important for dose retention, as such systems are prone to degradation of loaded molecules or leakage during storage (56). The cumulative in vitro release is shown in Figure 4. The study was conducted at two different pH levels (7.4 and 9.5) for 360 minutes, and the results were compared with the release profile of the pure Ibu solution (Ibu-Sol). A release of 42.3 ± 0.243% of Ibu was observed at pH 7.4 and 32.1 ± 0.481% at pH 9.5 from the Ibu-Ho@Cu/Al-LDH nanocomposite, while 93.4% of pure Ibu-Sol was released. Since Ibu is poorly soluble in water, a water/ethanol (1:1) solution was used as the receiving medium. The significant differences between pure Ibu-Sol and drug-loaded nanocomposites at both pH levels can be explained by the high compatibility of the drug with the receiving medium and the physical intercalation of Ibu into the interlayer spaces of the nanocomposite. The shift in drug release between the two pH levels might be attributed to the different presence of OH⁻ anions, which can alter the composition of the LDH nanocomposite and the characteristics of interlayer spaces. Fundamentally, the quantity and size/charge ratio of anions are crucial for a homogeneous balance between the positively charged layers. In the interlayer spaces, large-sized anions with low charge are unable to organize a host-guest relationship across host layers and guest species. In this context, anions containing long chains (fatty acid esters and long-chain alkyl carboxylates or sulfonates) can be ordered by several arrangements such as monolayer (parallel to the layers), parallel bilayer (tilted monolayers), or bilayers, leading to diversity in the interlayers (10). This functional diversity could be affected within different pH ranges. The working pH should not be lower than 4.0 due to the high fracture possibility of hydroxyl layers. However, LDH is soluble at low pH levels but remains stable at neutral and becomes substantially tightened at alkaline pH levels, expecting a controlled release profile (10, 57).
Kiani et al. demonstrated the pH sensitivity of Cu–Al LDH nanoparticles for loaded drug (doxorubicin) with higher released amounts in acidic pH ranges compared to neutral (21). Among different methods to overcome gastrointestinal (GI) barriers, pH-sensitive release mechanisms are emerging in oral administration. Although the oral route is recognized as the most common route of administration, molecules may encounter harsh conditions before reaching systemic circulation, particularly different pH ranges (pH 1–7) and destructive enzymes. In this respect, nanocomposites could solve such problems and provide a controlled release profile. By encapsulating LDHs within a pH-sensitive polymeric shell layer (particularly alkaline-solubles that are extensively used in enteric-coated dosage forms), core-shell nanocomposites could be achieved. Accordingly, the LDH nanocomposites would be entirely preserved to reach the intestine, providing a controlled release profile only after polymeric shell decomposition.
Apart from that, the pH sensitivity of drug delivery systems is desired in tumor-targeted therapies as cancerous cells are characterized by a high level of acidity (58). Hence, LDH nanocomposites could be used to provide a prompt release profile in such environments compared to normal ones. In this work, Ibu was chosen as a model pharmaceutical agent, which is commonly associated with adverse effects such as GI ulcers, renal features, and hepatic damage (59). Therefore, scientists have been encouraged to investigate alternative administration routes. Intercalating drugs in interlamellar spaces of nanocomposites not only allows for a controlled release profile and better therapeutic outcomes but also reduces the possibility of adverse effects, as less drug and dosing intervals would be required to achieve the same responses (22, 60).
In a study by Dasgupta, Mg/Al-LDH nanoparticles were synthesized for the intercalation of Ibu into the interlayer space by the co-precipitation method (22). Accordingly, the average particle size was measured to be 55 nm, and the release profile demonstrated to follow the first-order kinetic model in a 16-hour assessment. However, their cumulative release profile yielded 85% after 36 hours at pH 7.4. Wang et al. investigated the controlled release profile and antibacterial activity of synthesized graphene oxide–benzylpenicillin anion intercalated Mg/Al-LDH (GO–BP-LDH) nanohybrid films (61). According to their results, the synthesized GO-BP-LDH nanohybrid films not only provided a controlled release profile but also exhibited enhanced antibacterial activity, possibly due to the synergy of both graphene oxide and benzylpenicillin.
In the current study, different kinetic models were used to describe the release profile of the prepared nanocomposite. In this context, data were assessed, and the best-fitted kinetic model was identified based on the correlation coefficient value (Table 1). The validity of the selected kinetic model was also confirmed by AIC values. This mathematical method estimates the prediction errors and helps to assess the best fitting of a model to the data from which it was generated. Therefore, among models with close R² values, the model that shows a lower AIC value is more reliable. Accordingly, the Korsmeyer-Peppas model was chosen as the best curve fitting in both studied conditions (pH 7.4 and pH 9.5), which expresses the drug release pattern from a polymeric system regarding the exponent (N) of the equation. Theoretically, an N value less than 0.45 (N < 0.45) indicates that the release regime mainly follows hindered (or non-Gaussian) Fickian diffusion, which occurs when movements of loaded molecules are restricted by partially permeable barriers. An N value between 0.45 and 1 (0.45 < N < 1) indicates that loaded molecules would be released through anomalous transport (non-Fickian diffusion), which is attributed to further release mechanisms in addition to diffusion (62). In the current study, the latter kinetic mechanism (observed in the pH 7.4 release profile) may be ascribed to the lower presence of OH⁻ anions and less tightness of structure compared to alkaline conditions (pH 9.5).
| Variables | pH = 7.4 | pH = 9.5 | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | AIC | K | N | R2 | AIC | K | N | |
| Kinetics model | ||||||||
| Zero-order | 0.9868 | 4.9222 | 0.1351 | - | 0.9821 | 9.1765 | 0.0914 | - |
| First-order | 0.8003 | 1.7403 | 0.0017 | - | 0.8003 | 2.2838 | 0.0011 | - |
| Hixson-Crowell | 0.9421 | 8.0947 | 0.0024 | - | 0.9365 | 7.7792 | 0.0016 | - |
| Higuchi | 0.9747 | 9.5650 | 2.0534 | - | 0.9241 | 9.3043 | 1.3579 | - |
| Korsmeyer-Peppas | 0.9957 | 4.6872 | 0.0016 | 0.9736 | 0.9832 | 4.7609 | 0.0653 | 0.4161 |
Abbreviation: AIC, akaike information criterion.
Salguero et al. designed hybrid composite films using Zn-Al-LDH and hyaluronan (hyaluronic acid) as a delivery platform for intercalating ciprofloxacin (63). Their hybrid composite films, with potential use as an alternative approach for the prevention and treatment of wounds' opportunistic infections, represented a controlled release profile and best-fitted kinetics with Higuchi and Korsmeyer-Peppas models at pH 5.8 and 7.4, respectively. Therefore, after topical administration, this drug delivery system provides sustained release and maintains antibacterial activity at a suitable level along with the healing properties of hyaluronan.
Ranjbar and Namazi introduced Mg/Al-LDH@hydroxyapatite-doxorubicin coated magnetic Fe3O4-polyethylene glycol nanocomposite as a biocompatible and pH-sensitive system for targeted release of doxorubicin (64). The prepared nanocomposite demonstrated high cytotoxicity against MCF-7 cancer cells and a controlled release profile, which was found to be in good agreement with the Korsmeyer-Peppas model. However, the development of new drug delivery systems requires optimizing the release profile to reduce drug-related side effects. Therefore, the incorporation of therapeutic ingredients into nanocomposites is considered a viable approach to minimize adverse effects and address safety issues.
5. Conclusions
The continuous development of drug delivery approaches has led to significant advances in public health. In this context, nanoscience and nano-sized materials are drawing interest due to their promising applications in medicine. As a class of lamellar solids, LDHs can resemble conventional intercalating structures and qualitatively preserve loaded molecules during storage from degradation by harsh environments. These materials are typically prepared through the incorporation of an organic guest into a layered inorganic structure, yielding attractive hybrid organic/inorganic nanocomposites that can be used as carriers of different drugs and bioactive species. Intercalating drugs into the interlayer spaces of LDH nanoparticles can provide a controlled release profile and improve therapeutic responses while reducing adverse effects.
In the current study, we synthesized and evaluated the physicochemical characteristics of Cu/Al-LDH nanoparticles for controlled drug delivery. The prepared formulation exhibited good physicochemical properties with an average particle size of 123.7 nm and a PDI of 0.37. Further, SEM analysis revealed relatively uniform particles and suitable morphology. Additionally, this nanocomposite formulation could be used as an effective and low-cost adsorbent to remove different materials. The removal of pollutants, particularly dyes, is an essential step in wastewater treatment and plant health. Moreover, the Ho@Cu/Al-LDH nanocomposite also revealed good antioxidant properties, which are desirable for preserving oxidation-sensitive molecules during storage. It demonstrated high encapsulation efficiency with negligible alteration throughout the assessment time, as well as a pH-dependent release profile (42.3 ± 0.243% at pH 7.4 and 32.1 ± 0.481% at pH 9.5), following the Korsmeyer-Peppas kinetic model.
The incorporation of natural compounds into LDHs is no longer limited, introducing a suitable approach to minimize adverse effects. In the current study, Ibu was used as a model drug to define the potential applications of this system for controlled drug delivery. Honey was chosen in this system due to its high antioxidant capacity, which can help preserve the loaded molecules from harsh environments. However, the incorporation of natural-derived substances in delivery systems is mainly considered to be safe and biocompatible, providing access to a prolonged release profile. Despite considering the antimicrobial properties of honey, we aim to evaluate the potential antimicrobial properties of this drug delivery system, as well as exploit other pharmaceutical drugs such as antibiotics to achieve synergistic effects and further benefits. Nonetheless, cohort studies on possible toxicity and further applications of LDH nanocomposites should be conducted to assess their safety and feasibility before individual practices.